Molecule is one of the basic units of matter. It is the smallest particle into which a substance can be divided and still have the chemical identity of the original substance. If the substance were divided further, only molecular fragments or atoms of chemical elements would remain. For example, a drop of water contains billions of water molecules. If one of those water molecules were separated from the rest, it would still behave as water. But if that water molecule were divided, only atoms of the elements hydrogen and oxygen would remain.
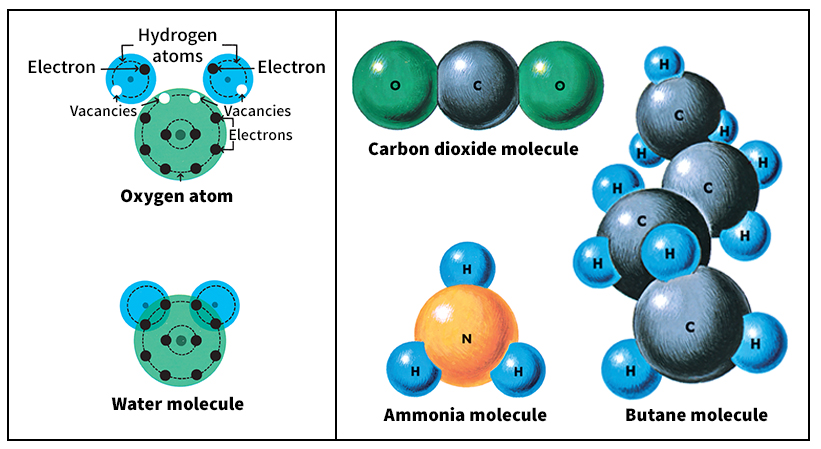
Individual molecules.
Molecules are made up of atoms held together in certain arrangements. Scientists use chemical formulas to show the composition of molecules. For example, a water molecule consists of two hydrogen atoms and one oxygen atom, and it has the formula H2O. A molecule’s size and shape depends on the size and number of its atoms. A molecule that consists of two atoms, such as nitric oxide (NO), is called a diatomic molecule. A molecule made up of three atoms, such as water, is called a triatomic molecule. A large molecule, such as DNA, can contain millions of atoms.
Atoms link together in molecules through strong attractive forces called bonds (see Bond). The shape of a molecule depends upon two factors: (1) The atoms tend to take up positions relative to one another such that the bonds formed are the strongest of all the bonds that this particular group of atoms could form. (2) Atoms that are not bonded to each other tend to move far apart. For example, an ammonia molecule has the shape of a tetrahedron (a pyramidlike figure with four faces). It consists of three hydrogen atoms attached to a nitrogen atom. Normal butane molecules have 4 carbon atoms arranged in a zigzag chain with 10 hydrogen atoms attached. Large protein molecules can form long spiral chains.
The mass (amount of matter) of a molecule is indicated by its relative molecular mass. You can find the relative molecular mass of a molecule by adding the relative atomic masses of all the atoms in the molecule. An atom’s relative atomic mass equals its mass divided by 1/12 of the mass of an atom of carbon 12, the most abundant form of carbon.
Suppose, for example, you wished to calculate the relative molecular mass of a molecule of carbon dioxide (CO2) that consists of one atom of carbon 12 and two atoms of the most abundant form of oxygen. The relative atomic mass of the carbon atom would be exactly 12, and the relative atomic mass of each of the oxygen atoms, rounded to five figures, would be 15.995. Your calculation would be 12.000 + 15.995 + 15.995 = 43.990. A molecule’s mass can also be measured with an instrument called a mass spectrometer. Carbon dioxide has a molecular mass of about 44.
Polar molecules and ionic substances.
Each atom in a molecule consists of a positively charged nucleus surrounded by a cloud of negatively charged electrons. In a neutral molecule, the positive and negative charges are evenly balanced throughout the molecule. In polar molecules, the charges are not evenly balanced. In a polar molecule, more positive charge collects at one location in the molecule and more negative charge collects at a different location. Some molecules are magnetic because of the way the electrons are unevenly distributed within the molecule.
Almost all gases, most common liquids, and many solids are made up of neutral or polar molecules. But some substances are made up of units called ions (atoms or groups of atoms with a positive or a negative charge). These substances are called ionic substances.
Salts are examples of ionic substances. For example, sodium chloride, common table salt, consists of positive sodium ions and negative chloride ions. Electric forces among the ions hold them together in a regular framework. Metals are also different from molecular substances. In addition to positive ions, metals consist of a large number of electrons that move about freely throughout the metal.
Molecules and matter.
Molecules are held together in a group by electrical forces called van der Waals forces. These forces are usually weaker than those that hold a molecule itself together. The force between molecules depends on how far apart they are. When two molecules are widely separated, they attract each other. When they come very close together, they repel each other.
In a solid, the molecules are so arranged that the forces which attract and repel are balanced. The molecules vibrate about these positions of balance, but they do not have enough energy to move to different parts of the solid. As the temperature of a solid is raised, the molecules vibrate more strongly. When the van der Waals forces can no longer hold the molecules in place, the solid melts.
In a liquid, the molecules move about easily, but they still have some attractive force on one another. These forces are strong enough to keep the liquid together. Certain organic compounds called liquid crystals have properties of both liquids and solids. Within a particular temperature range, such a compound flows like a liquid, but has a more ordered molecular arrangement. Its molecules line up side by side and form tiny groups or clusters that slide past one another in certain directions. See Liquid crystal.
In a gas, the molecules move about so fast that the attractive forces have little effect on them. When two molecules in a gas collide, the repelling force sends them apart again. Therefore, gas molecules fill a container completely, because they move freely through all the space available.
Most substances can be changed into solids, liquids, or gases by either raising or lowering their temperatures. The temperature at which these changes occur–and also other characteristics of a substance–depends on the size, shape, and mass of the molecules and also on the strength of the van der Waals forces between them.
Under certain conditions, two molecules may collide with enough energy to react and form one or more new molecules. The process by which many small molecules combine chemically to produce a large molecule is called polymerization. Molecules can also break down into smaller molecules. Causes of molecular disintegration include ultraviolet light, fast-moving electrons, and nuclear radiation.
Studying molecules.
Scientists can study some molecules directly with an electron microscope. This method provides a picture of a molecule, but the picture is often too blurred to see fine details. A scanning tunneling microscope produces an image of some individual atoms in a solid substance. Scientists also study molecules indirectly. For example, they study solids by X-ray diffraction. The way a solid deflects X rays tells them about the size, shape, and arrangement of its molecules. Scientists also use neutron diffraction and electron diffraction to study solids. They pass a beam of neutrons (uncharged particles) or electrons through a solid, and observe how the beam is affected. Electron diffraction can also be used to study gases.
Scientists also learn about molecules by studying the way they absorb or give off light. Each kind of molecule absorbs or gives off certain colors of light. This group of colors makes up the molecule’s spectrum. By studying the spectrum of a substance, scientists can find the sizes and shapes of its molecules, the strength of the forces that hold the atoms together in the molecules, and the way the electrons move about in the molecules.
