Neon is a chemical element that makes up about 1 part per 65,000 in Earth’s atmosphere. The British chemists Sir William Ramsay and Morris W. Travers discovered the element in the atmosphere while they were studying liquid air in 1898. Ramsay had predicted the existence of this gas in 1897. Ramsay and Travers named the gas neon, for the Greek word that means new.

Neon is used chiefly for filling lamps and luminous sign tubes. Its usual color in lamps is bright reddish-orange. The addition of a few drops of mercury makes the light a brilliant blue.
Many airplane beacons use neon light because it can penetrate fog. A number of airplane pilots have reported that neon beacons were visible for 20 miles (32 kilometers) when it was impossible for them to see other kinds of lights.
Neon lamps are made by removing the air from glass tubes and then filling them with neon gas. When about 15,000 volts of electricity are applied to the tube, an electric discharge occurs, and the tube glows fiery reddish-orange. Instead of a filament, a neon tube has two electrodes sealed within it. The neon forms a luminous band between these electrodes.
Commercially, neon is obtained as a by-product of liquid air manufacture. Neon liquefies under normal pressure at –246.048 °C, and it freezes at –248.67 °C. When air is liquefied at about –200 °C, neon is left behind as a gas. Neon is expensive, but very little is needed for lamps. Signs use 1 quart per 200 to 300 feet (1 liter per 64 to 97 meters) of tubing. Liquid neon is often used as a low-temperature refrigerant (cooling agent).
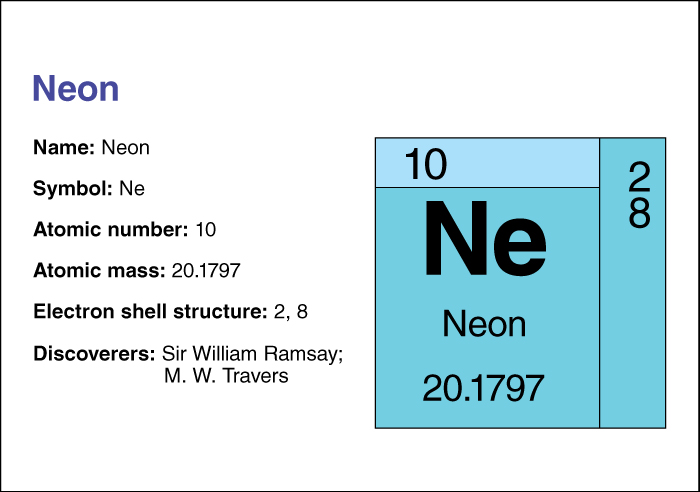
Neon is a colorless, odorless gas. It does not react readily with other substances, though it may form a compound with fluorine. Neon is classed as a noble gas. Its symbol is Ne. It has an atomic number (number of protons in its nucleus) of 10. Its relative atomic mass is 20.1797. An element’s relative atomic mass equals its mass (amount of matter) divided by 1/12 of the mass of carbon 12, the most abundant form of carbon. For information on the position of neon on the periodic table, see the article Periodic table.
