Neptunium << nehp TOO nee uhm >> (chemical symbol, Np), is a radioactive chemical element . Its atomic number (the number of protons in its nucleus) is 93. Almost all neptunium is created in nuclear reactors. Traces of the element exist in uranium ores. Neptunium was named for the planet Neptune. It follows uranium in the periodic table of the elements, just as Neptune follows Uranus in the solar system.
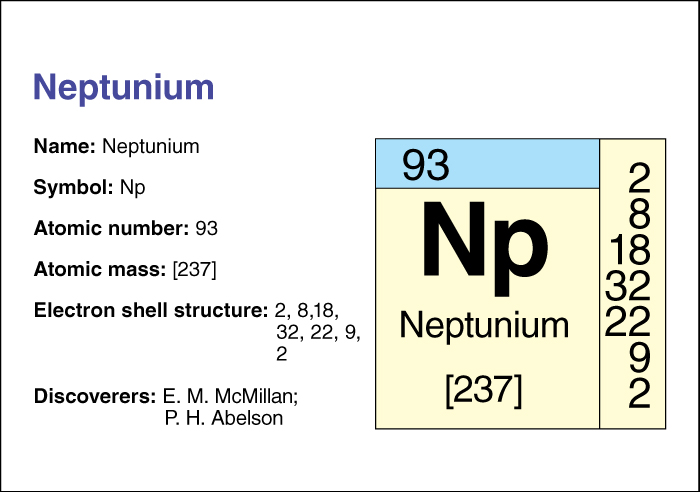
There are 17 known forms or isotopes of neptunium. Different isotopes of an element have the same number of protons but different numbers of neutrons. Neptunium-237, the most stable isotope of neptunium, has 144 neutrons and an atomic mass number (total number of protons and neutrons) of 237. Isotopes of neptunium undergo radioactive decay—that is, their nuclei break apart. As a result, their atoms turn into atoms of another isotope. The time that it takes half of a sample of an isotope to decay into another isotope is called its half-life. Neptunium-237 has a half-life of 2.14 million years.
Chemists classify neptunium in the actinide group. For information on the position of neptunium on the periodic table, see the article Periodic table .
Neptunium was discovered by the American physicists Edwin M. McMillan and P. H. Abelson at the University of California in 1940. They bombarded the isotope uranium-238 with neutrons to create the isotope neptunium-239. Neptunium-239 has a half-life of 2.4 days and decays to form the isotope plutonium-239, which can be used in nuclear reactors and nuclear weapons. Neptunium metal has a density of 20.25 grams per cubic centimeter at 20 °C. It melts at 639 °C.
See also McMillan, Edwin Mattison .
