Neutralization << `noo` truh luh ZAY shuhn or `nyoo` truh ly ZAY shuhn >> is a chemical reaction in which an acid and a base form a salt. If the reaction is complete—and the acid and base are both strong—the final salt is neutral (neither acidic nor basic).
Strong acids and bases in water solution ionize (break down) completely into positive and negative particles called ions. This reaction is shown below for hydrochloric acid (HCl) and sodium hydroxide (NaOH).
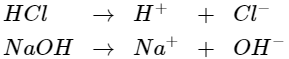
Weak acids and bases also break down in water. But they do not ionize completely.
When the acid and base react together, the hydroxide (OH–) ion from the base combines with the hydrogen (H+) ion from the acid to form water (H2O).
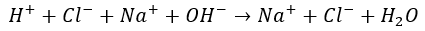
The two remaining ions form a salt that may either precipitate (drop out of the solution as a solid) or stay in solution as ions. If the water is evaporated, the salt can be recovered in crystal form. When neutralization occurs in water solution, it is defined as the reaction between hydroxide and hydrogen ions to form water. Chemists can tell when a neutralization reaction is complete by using indicators, such as litmus.
