Niobium << ny OH bee uhm >> also called columbium, is a soft, silver-white or gray metallic element. In nature, it occurs chiefly combined with tantalum, another rare metal. Manufacturers alloy much niobium with nickel and steel to make strong structural materials that are resistant to high temperatures. Niobium is also used in the cores of certain nuclear reactors because it allows neutrons to penetrate easily and can withstand high-temperature reactor coolants. At extremely low temperatures, niobium becomes a superconductor, and so it is used in making superconducting magnets (see Superconductivity).
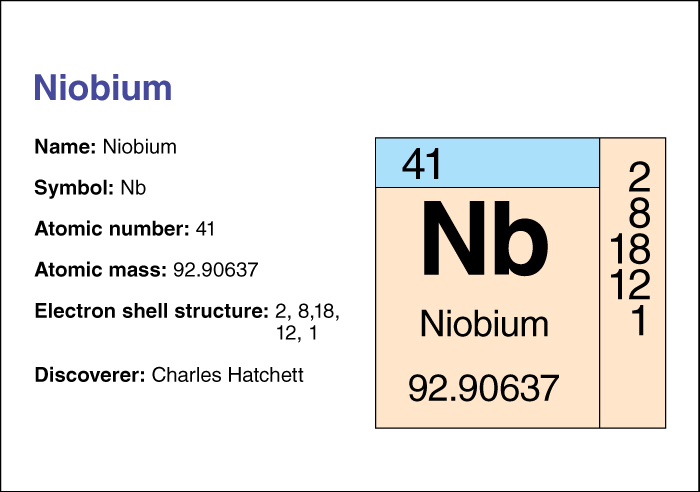
Niobium has the chemical symbol Nb. Its atomic number (number of protons in its nucleus) is 41. Its relative atomic mass is 92.90638. An element’s relative atomic mass equals its mass (amount of matter) divided by 1/12 of the mass of carbon 12, the most abundant form of carbon. Niobium melts at 2468 plus or minus 10 °C and boils at 4742 °C. Niobium has a density of 8.57 grams per cubic centimeter at 20 °C (see Density). Charles Hatchett, a British chemist, discovered niobium in 1801.
Chemists classify niobium as a transition metal. For information on the position of niobium on the periodic table, see the article Periodic table.
See also Element, Chemical (Periodic table).
