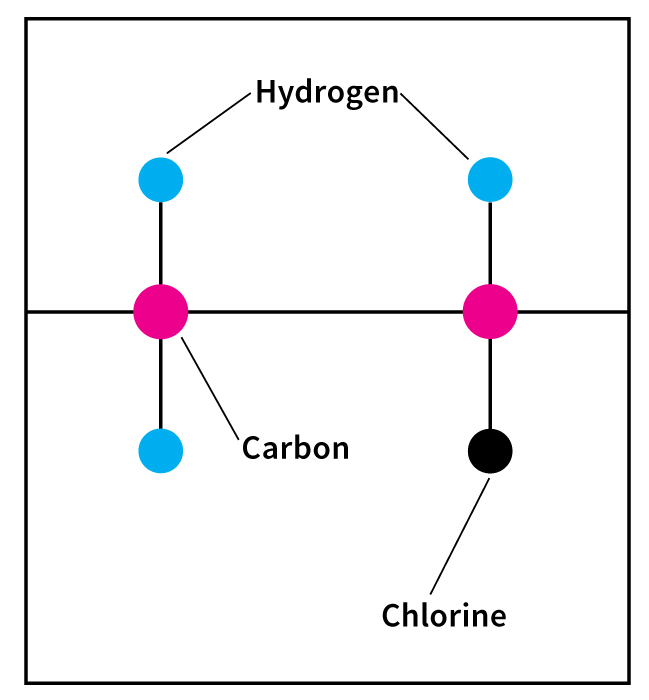
Petrochemicals, << `peht` roh KEHM uh kuhlz, >> are chemicals made from petroleum or natural gas. They are among the most important materials used in industry. Manufacturers use petrochemicals in making such products as detergents, fertilizer, medicines, paint, plastics, synthetic fibers, and synthetic rubber.
The basic materials of the chemical industry are the primary petrochemicals. They may be divided into three major groups, according to their chemical structure: (1) olefins, (2) aromatics, and (3) synthesis gas.
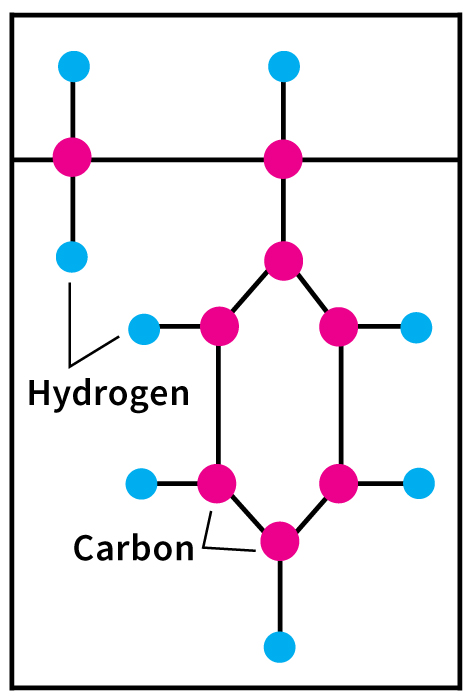
Important olefins include ethylene, propylene, and butadiene. Ethylene and propylene serve as important sources of industrial chemicals and plastics products. Butadiene is used in making synthetic rubber.
The chief aromatic petrochemicals include benzene, toluene, and xylenes. Benzene is used in the manufacture of dyes and synthetic detergents. Toluene is used in making explosives. Manufacturers use xylenes in making plastics and synthetic fibers.
Synthesis gas is a mixture of carbon monoxide and hydrogen. The petrochemicals ammonia and methanol are made from synthesis gas. Ammonia is used in making fertilizer and explosives. Methanol serves as a source for other chemicals.
How petrochemicals are made.
Petroleum and natural gas consist chiefly of compounds of the elements hydrogen and carbon. These compounds are called hydrocarbons. Most petrochemicals contain carbon that comes from such hydrocarbon compounds.
One important method of producing olefin and aromatic primary petrochemicals is a process called steam cracking. In this process, hydrocarbons separated from natural gas or crude oil are mixed with steam in a tubular furnace and quickly heated to between 1,470 and 1,650 °F (800 and 900 °C). The hydrocarbons are thus broken down into simpler compounds, which then combine to form the desired petrochemicals. Primary petrochemicals, especially aromatics, are produced as by-products of petroleum refining.
Complex petrochemicals are made by combining two or more primary petrochemicals. For example, ethylene and benzene can be combined to form ethylbenzene, used to make synthetic rubber. Other complex petrochemicals include polyethylene, polypropylene, and polyvinyl chloride. Manufacturers use these petrochemicals in producing such plastics goods as car parts, electrical insulation, leatherlike clothing and luggage, and squeeze bottles.
History.
The first chemical to be made from petroleum or natural gas was produced from natural gas in the United States in 1872. This chemical, carbon black, is now used as a reinforcing material in tires.
The widespread use of oil and gas to make chemicals began during the 1920’s. Until that time, chemical companies used coal as a source of many chemicals. Then the companies began using petroleum and natural gas to produce the same chemicals because oil and gas were cheaper and easier to obtain than coal. Petrochemicals enabled manufacturers to cheaply produce such materials as plastics and synthetic fibers.
The use of petrochemicals increased rapidly during World War II (1939-1945). The armed forces used many products made from petrochemicals, including explosives and synthetic rubber.
Until the late 1900’s, the United States, Japan, and the nations of Western Europe dominated the petrochemical industry. Many nations in the Middle East and Asia then began producing petrochemicals for export rather than merely exporting crude oil and natural gas. Developing and operating petrochemical facilities has helped these countries improve living standards for their people.