Promethium, << pruh MEE thee uhm >> (chemical symbol Pm), is a chemical element and one of the lanthanide metals. Some scientists think promethium may occur naturally on Earth in trace amounts, and astronomers have detected it in a star. But scientists have been able to produce it only artificially. It exists as radioactive isotopes among the fission products of uranium, thorium, and plutonium. An element’s isotopes have the same number of protons but different numbers of neutrons. Promethium was first isolated in 1945, by the American chemists J. A. Marinsky, Lawrence E. Glendenin, and Charles D. Coryell.
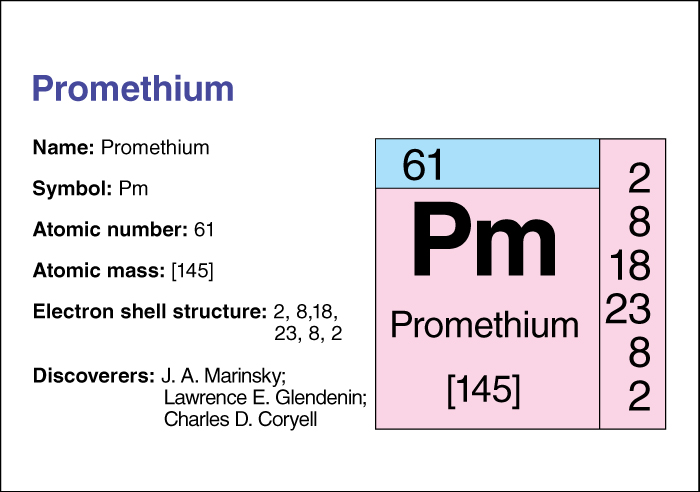
Promethium’s atomic number (number of protons in its nucleus) is 61. Its most stable isotope has an atomic mass number (total number of protons and neutrons) of 145, and its most abundant isotope has an atomic mass number of 147. The melting point of promethium is 1042 °C, and its boiling point is estimated to be about 3000 °C. For information on the position of promethium on the periodic table, see the article Periodic table . See also Rare earth .
