Proton << PROH ton >> is a positively charged subatomic particle. A single proton constitutes the nucleus of an ordinary hydrogen atom. Protons, together with other subatomic particles called neutrons, make up the nuclei of all other atoms. All atoms of the same chemical element have the same number of protons. The number of protons in the atoms is called the atomic number of the element.
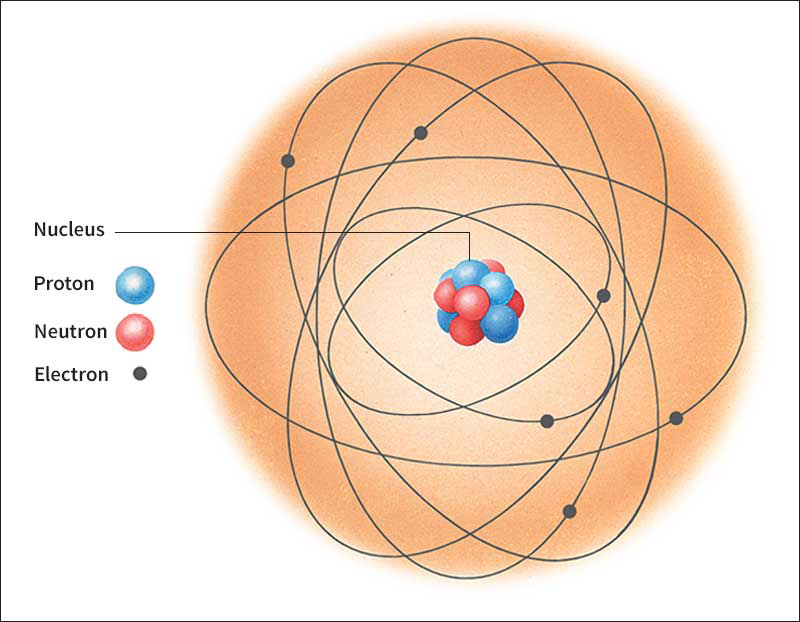
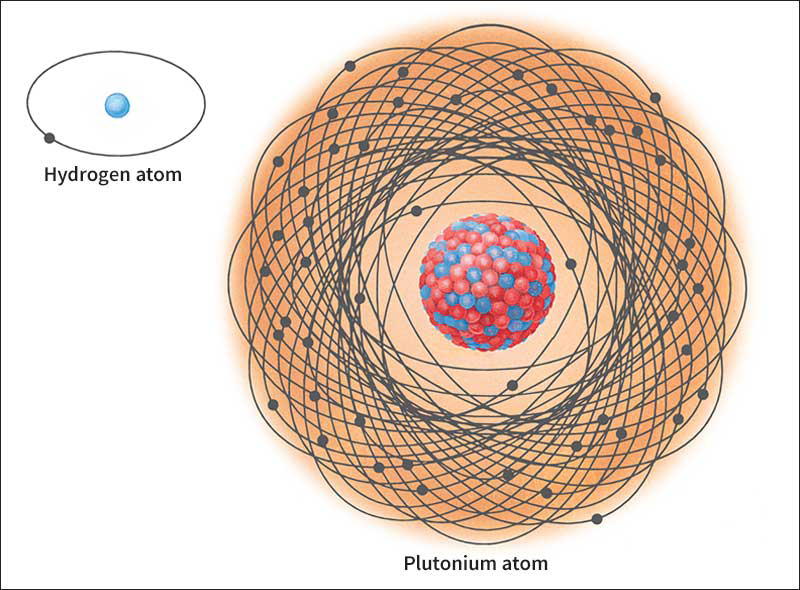
Ordinarily, an atom has an equal number of protons and electrons, negatively charged particles that surround the nucleus. Each proton carries one unit of positive charge, and each electron carries one unit of negative charge. As a result, the atom is electrically neutral.
Protons are made up of fundamental particles called quarks. A proton has a diameter of approximately one millionth of a nanometer. One nanometer equals one millionth of a millimeter (1/25,400,000 inch). The mass of a proton in grams may be written with a decimal point followed by 23 zeros and a 2.
The proton was first identified by the German physicist Wilhelm Wien in 1902. The British physicist Sir Joseph J. Thomson proved the identity of the proton in 1906.
