Battery is a device that stores energy as chemical energy and releases it as electric energy. Chemical reactions in a battery create an electric current. Electric currents from batteries can power many devices, from tiny portable electronics to large automobiles.

Batteries come in hundreds of sizes and use many different combinations of chemicals. They can be grouped into two main classes, primary batteries and secondary batteries. Primary batteries cannot be recharged. Secondary batteries can be recharged multiple times. But they usually store less energy than do primary batteries of the same size. Secondary batteries are used in computers, cell phones, power tools, and vehicles.
How batteries work
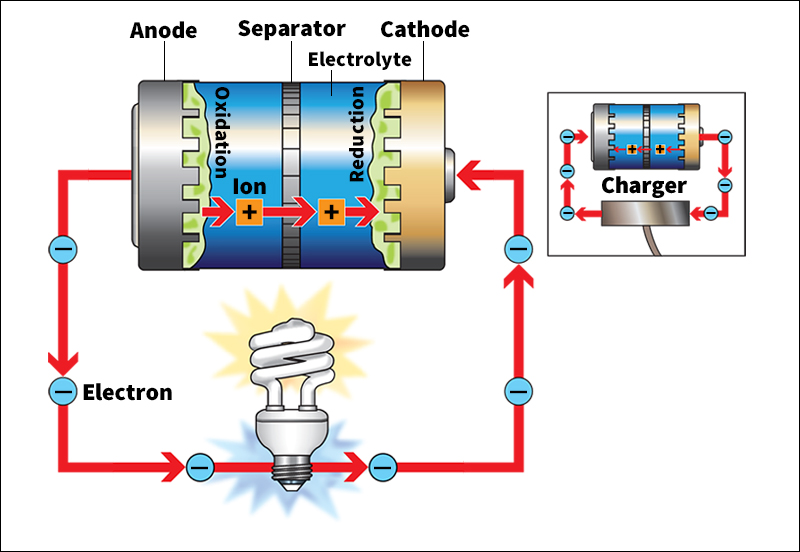
An electric current consists of tiny particles called electrons. Usually, electrons form part of atoms. A battery works by separating electrons from atoms in the battery’s material and “pushing” them into an electric circuit. A circuit is a looping path through which electric current travels. The flow of electrons through the circuit forms a current. Battery-powered devices, in turn, can be connected to the circuit. These devices change the electric energy of the current into other useful forms of energy, such as light, sound, and motion.
Batteries separate electrons from atoms and “push” them by means of chemical reactions at two electrodes, also called poles. The first electrode, called the anode, is generally marked with a minus sign (−). It releases electrons into the circuit. The electrons flow around the circuit and power any connected devices. The electrons reenter the battery through the second electrode, the cathode. It is generally marked with a plus sign (+).
Electric charges.
A battery’s operation relies on the principle of electric charge. An atom consists of a nucleus (core) surrounded by a whirling cloud of electrons. Particles in the nucleus called protons have positive charges. Electrons have negative charges.
Usually, an atom has no overall charge, because the positive charges of its protons balance out the negative charges of its electrons. But atoms or molecules may lose or gain electrons. They then become charged particles called ions. Ions with extra electrons have negative charges. Ions with missing electrons have positive charges.
Positive and negative charges behave much like the north and south poles of magnets. Two opposite charges will attract each other. Thus, the negatively charged electrons usually remain close to their atom’s positively charged nucleus. On the other hand, two like charges will repel (push away from) each other. For this reason, electrons flow away from areas of negative charge. Positively charged ions, likewise, flow away from areas of positive charge.
A battery, together with a circuit, forms a complete loop of moving charges. The circuit conducts negative charges—electrons—from the anode, through any devices, and back to the cathode. Meanwhile, free positive charges—in the form of ions—move from anode to cathode inside the battery, essentially moving in the direction opposite from the electrons. Chemical reactions at the anode and cathode provide the energy to “push” the charges around the loop.
Battery structure.
A battery consists of one or more electrochemical cells. A cell is filled with an electrolyte that separates an anode and a cathode. Electrolytes are acids, bases, or chemical salts. Wet cells use liquid electrolytes. Dry cells use gel or pastelike electrolytes. The electrolyte conducts ions. It also reacts chemically with materials in the cathode and anode. Electrodes and electrolytes are made of a variety of chemical elements and compounds, depending on the type of battery.
Terminals are structures on the exterior of the battery. They connect the electrodes to a circuit.
Most cells also include a separator, a porous (hole-filled) barrier between the two electrodes. The separator prevents the anode and cathode from touching, while allowing the electrolyte to pass through. It ensures that the electrons flow “the long way” around the external circuit by preventing them from moving directly from the anode to the cathode. If electrons do move directly from anode to cathode, their path forms an internal short circuit. It renders the battery unusable.
Half-cell reactions.
All types of battery create electric current through two chemical reactions called half-cell reactions. They are (1) the oxidation reaction and (2) the reduction reaction.
The oxidation reaction takes place at the anode. It causes atoms at the anode to lose electrons. The electrons flow out to the circuit. The reduction reaction takes place at the cathode, as electrons flow into the cathode material from the circuit. The overall combination of reactions generally produces stable, uncharged material.
In primary batteries, the half-cell reactions cannot be reversed, or cannot be reversed easily. But in secondary batteries, the reactions can run in reverse. A charging device forces electric current to flow the opposite direction through the circuit, from the cathode to the anode. The resulting chemical reactions restore the battery’s active chemicals to an energy-producing state.
Types of batteries
Batteries are typically named for the type of materials used in the electrodes and in the electrolyte. These materials help determine the voltage of the battery. The voltage is the force with which an electric current is “pushed” through a circuit. The arrangement of the electrodes and electrolyte also varies widely among different types of batteries.
Primary batteries
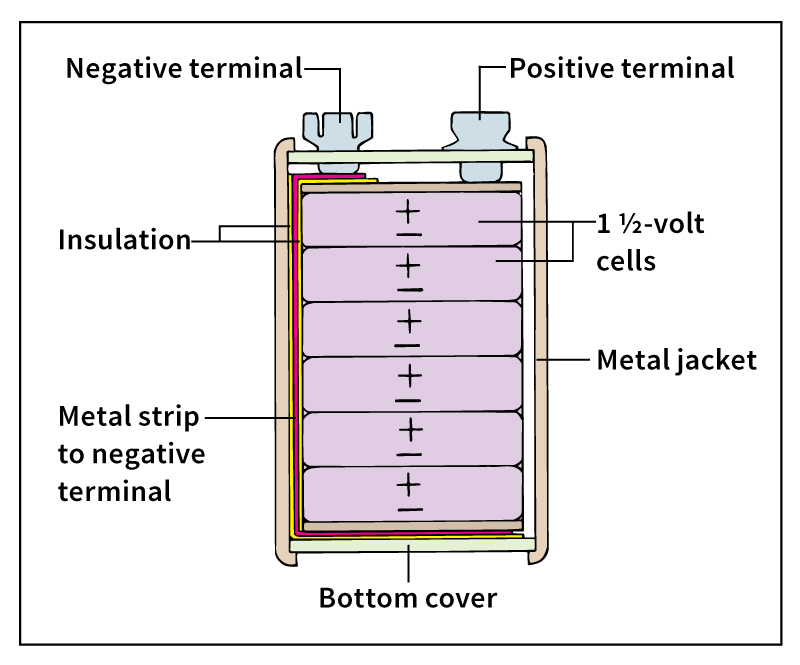
cannot be recharged, although they typically store more energy than do similarly sized secondary batteries. Primary batteries include alkaline and carbon-zinc batteries. Primary batteries are widely used to power small electronic devices and toys.
Alkaline primary batteries
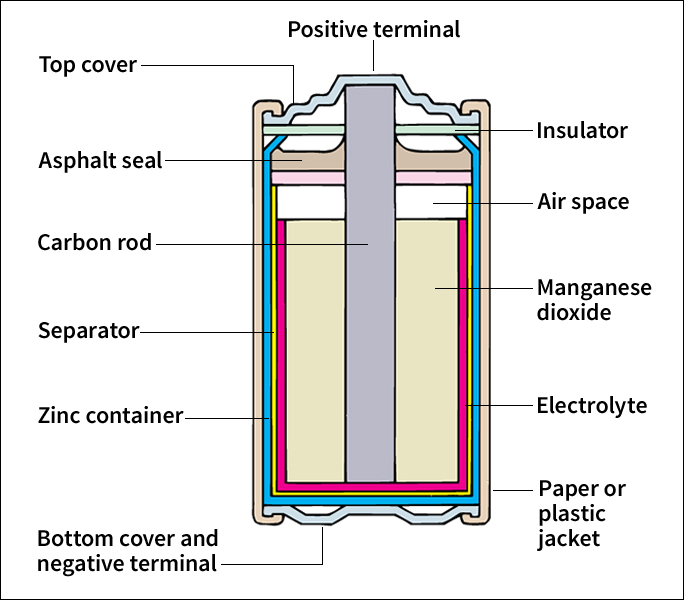
use a base for an electrolyte. The word alkaline means base. The most common alkaline primary batteries use a water-based potassium hydroxide electrolyte, a cathode of carbon and manganese dioxide, and a zinc anode. In some cases, silver oxide is used in the cathode for a costlier battery that produces more energy.
Carbon-zinc batteries
use a chemistry similar to that of alkaline batteries, but they have a dissolved salt—usually ammonium chloride—as the electrolyte instead of a base. Zinc serves as the anode. The cathode contains carbon and manganese dioxide.
Secondary batteries
can be recharged with an electric current. They include lead-acid, metal-hydride, and lithium-ion batteries.
Lead-acid batteries
are used widely in automobiles with conventional internal-combustion engines. These batteries do not actually power the motor, but they provide electric current to the automobile’s ignition system and electronics. A 12-volt automobile lead-acid battery includes six 2-volt cells inside a plastic container. Each cell has two electrode structures called grids. Both electrode structures are made of lead-containing alloys (combinations of a metal and one or more other elements). The electrodes are immersed in a sulfuric acid electrolyte.
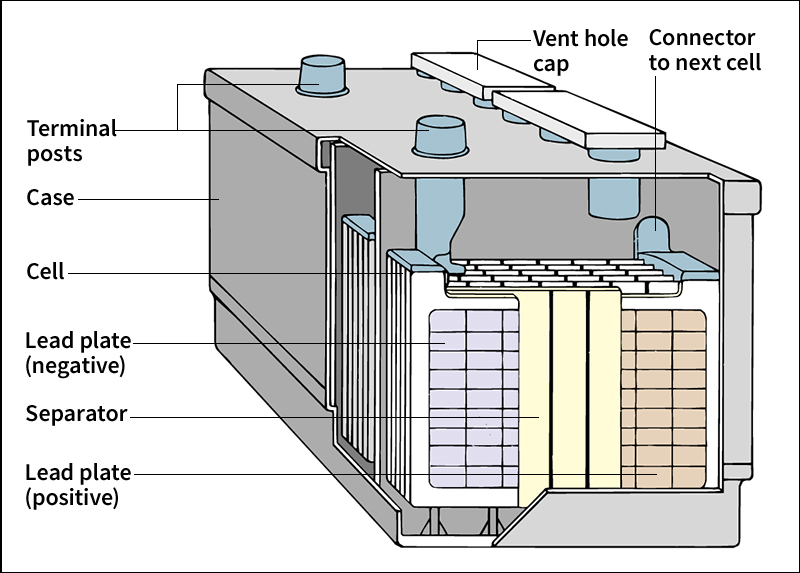
In use, chemical reactions at both electrodes produce lead sulfate. The lead of the anode reacts with negatively charged sulfate ions in the electrolyte to produce lead sulfate. At the cathode, lead dioxide reacts with the electrolyte to produce lead sulfate. The reactions use up positive ions in the electrolyte, making it less acidic and more like ordinary water.
Modern automobile lead-acid storage batteries are sealed except for a gas-venting safety valve. The seal prevents water loss from evaporation. They use grids made of lead-calcium-tin or similar alloys, which last a long time. The alloy also helps minimize water loss.
Metal hydride batteries,
also called nickel metal hydride batteries, use a special metal alloy that absorbs hydrogen as the anode’s active material. Metal hydride battery alloys can absorb about a thousand times their own volume of hydrogen. Hydrogen is the lightest atom, containing just one electron and one proton. During use, electrons from the anode’s hydrogen flow out to the circuit. Protons from the hydrogen enter the electrolyte and form water.
All metal hydride batteries use nickel hydroxide cathodes and water-based potassium hydroxide electrolytes. The anode is an alloy that can be made of various metals. Metal hydride batteries are used in camcorders, cellular telephones, computers, and hybrid automobiles. A metal hydride cell typically produces about 1.2 volts when operating.
Lithium-ion batteries
use the element lithium as the charge-carrying ion in the electrolyte. Such batteries have stable, reversible reactions. They also typically produce nearly 4 volts when operating and are thus more powerful than other secondary batteries. Lithium-ion batteries are used in many electronics. In the late 1900’s and early 2000’s, manufacturers began developing large lithium-ion batteries that could power electric vehicle motors.

All lithium-ion batteries work by moving lithium ions from one electrode to another. During use, the lithium ions move from the anode to the cathode. During recharging, the lithium ions move in the opposite direction. Aside from lithium, the materials in a lithium-ion battery vary.
Common cathode materials in lithium-ion batteries include compounds of lithium, oxygen, and certain metals. Lithium metal oxide cathodes contain oxygen and use cobalt, nickel, manganese, aluminum, or chromium as the metal. Lithium metal phosphate cathodes use iron or manganese as the metal, along with a phosphate. Phosphates are chemical structures that contain one phosphorus atom and four oxygen atoms.
Lithium-ion battery anodes are typically graphite, a form of the element carbon. Some anodes use other materials, such as alloys containing tin and silicon.
The electrolytes in lithium-ion batteries are dissolved salts that contain lithium. The salts are not dissolved in water, as in many other batteries. Instead, they are dissolved in an organic (carbon-based) solvent, such as ethylene carbonate, propylene carbonate, or dimethyl carbonate. Such electrolytes, unlike water-based electrolytes, generally remain stable at high voltages. However, they have the disadvantage of poorly conducting ions.
To compensate for the electrolyte’s poor conductivity, the electrodes are spread out in microscopically thin layers. Thus, the lithium ions must only travel a microscopically short distance through the poorly conducting electrolyte. In common designs, thin sheets of alternating electrode structures are wound into rolls or layered into pouches. The rolls or pouches are then sealed.
History
Archaeologists have discovered ancient clay jars in what is now Iraq that may have functioned as primitive battery cells. One jar, the so-called “Baghdad Battery,” dates from as early as 250 B.C. It contained an iron rod and a copper cylinder that could have functioned as electrodes. The jar also showed evidence of corrosion from an acid. Such an acid may have been used as an electrolyte.
The first documented working cell was made around 1800 by Alessandro Volta, an Italian scientist. Volta prepared several variations of the device, which consisted of layers of metal plates. Pairs of the plates were separated by cardboard or a woven cloth soaked in solution. The device was known as a pile.
In the early 1830’s, an English scientist named Michael Faraday showed that oxidation and reduction reactions create the force that pushes an electric current. Faraday also helped coin the terms anode, cathode, anion (negative ion) and cation (positive ion). Several years later, more practical batteries were developed. In 1836, John F. Daniell, an English chemist, introduced a more efficient primary cell that used copper and zinc. In 1859, the French physicist Gaston Plante invented the first secondary battery. It used lead-acid chemistry.
The development of lithium-ion batteries began in the 1970’s. Sony, a Japanese electronics company, produced the first commercial lithium-ion batteries in 1990. In the early 2000’s, such devices as portable media players and cameras increasingly came equipped with their own rechargeable batteries.
