Sulfur is a yellow, nonmetallic chemical element. Sulfur is found in many parts of the world. It has been used for hundreds of years. The ancient Greeks and Romans used sulfur as a cleanser, bleach, and medicine. The element was later important as one of the main ingredients in gunpowder. Today, sulfur is used in a variety of products and industrial processes.

Sulfur occurs alone in nature. It is also found in coal, crude oil, natural gas, oil shales, and many minerals. The most abundant of all sulfur minerals is a compound of sulfur and iron called pyrite. The atmosphere of Venus contains sulfur. Some scientists believe the core of Mars consists of pure iron sulfide, another compound of sulfur and iron. Astronomers have found sulfur compounds in interstellar clouds and in meteorites.
All plants and animals need small amounts of sulfur to live. Plants obtain sulfur from the soil. Many foods from plants are rich in sulfur compounds. These foods include cabbage, garlic, onions, and soybean flour. Methionine, a substance required in the human diet, also contains sulfur. It is found in such foods as eggs, dairy products, and meats.
Uses.
Almost all sulfur produced today is used to prepare sulfuric acid, a sulfur compound. This substance is one of the most important commercial chemicals. Manufacturers use sulfuric acid to make such products as dyes, paints, paper, and textiles. Sulfuric acid is an ingredient of a number of industrial chemicals. The compound is also used in the production of metals and in petroleum refining.
Products that contain sulfur—but not sulfuric acid—include fertilizers and some types of explosives, fungicides, and insecticides. Other sulfur-containing products include rubber, shampoos, storage batteries, and chemicals used in developing photographic film. Sulfur is also an ingredient in many medicines. It may be used in highway construction instead of asphalt.
Properties.
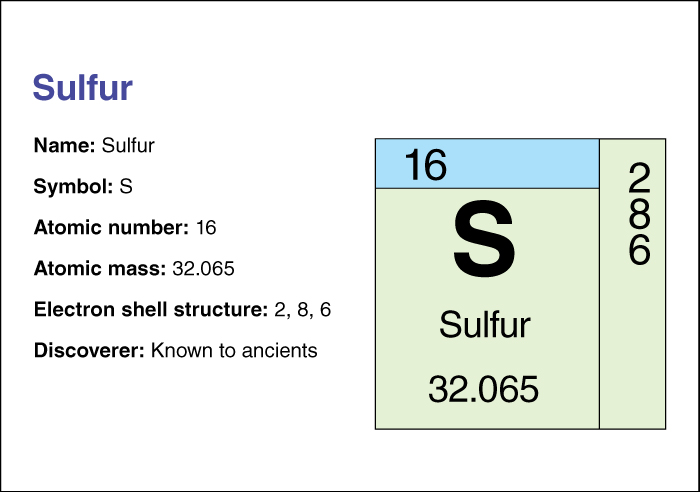
Sulfur has no taste or odor. Its atomic number (number of protons in its nucleus) is 16. Its relative atomic mass is 32.065. An element’s relative atomic mass equals its mass (amount of matter) divided by 1/12 of the mass of carbon 12, the most abundant form of carbon. The chemical symbol for sulfur is S. Sulfur melts at 120 °C if it is heated slowly. It melts at 113 °C if it is heated rapidly. It boils at 444.6 °C. Between 150 °C and 444.6 °C, sulfur goes through many changes in density and color as it takes on different forms called allotropes. For information on the position of sulfur on the periodic table, see the article Periodic table .
Sulfur is a reactive element. At 250 °C, it ignites with air. As it burns, it combines with oxygen to form sulfur dioxide, a colorless gas. Large amounts of sulfur dioxide can be found in the air of many densely populated areas. This gas has been associated with respiratory disorders and damage to buildings. It also contributes to a type of precipitation called acid rain (see Acid rain ). Much of the sulfur dioxide in the air is formed when coal that contains sulfur is burned. In the United States and certain other countries, environmental protection laws limit the amount of sulfur that can be emitted by coal-burning power plants.
Forms.
Sulfur exists in several allotropes. The most common allotrope is orthorhombic sulfur, also called rhombic sulfur. It is a lemon-yellow, crystalline material that is stable at room temperature. Monoclinic sulfur, or prismatic sulfur, is stable only between 94 and 120 °C. It occurs in long, almost colorless, needlelike crystals. Amorphous sulfur, also called plastic sulfur, is soft and sticky and stretches like rubber. Both monoclinic sulfur and amorphous sulfur change to the orthorhombic form at room temperature.
Orthorhombic sulfur is prepared in several ways for commercial use. For example, fine grains of sulfur are produced when sulfur vapor condenses. These grains are called flowers of sulfur because they occur in flowerlike patterns. Roll sulfur is made by hardening liquid sulfur in cylinder-shaped molds. Sulfur nuggets are prepared by spraying molten sulfur into a water bath.
How sulfur is obtained.
Before 1900, many industries obtained sulfur from volcanic deposits, sulfur mines in Sicily, and roasted pyrites. The United States led the world in sulfur production during the 1900’s. In the early 2000’s, the United States and Canada ranked as the leading producers of sulfur. Other sulfur-producing countries included China, Japan, and Russia.
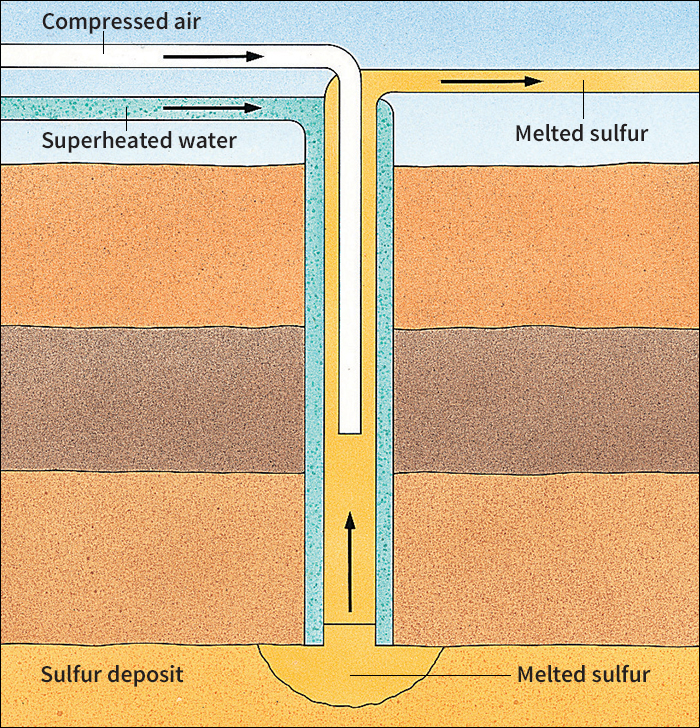
From 1900 until the mid-1950’s, the chief process used to produce sulfur was the Frasch method. In 1891, Herman Frasch, an American chemical engineer, discovered that sulfur could be melted underground with superheated steam. In the Frasch process, water is heated under pressure to a temperature above sulfur’s melting point. Pumps force the water into the ground, where it melts sulfur into a frothy liquid. Compressed air then forces the liquid sulfur to the surface. Most sulfur produced by the Frasch method is about 99.5 to 99.9 percent pure. See Mining (Pumping methods) .
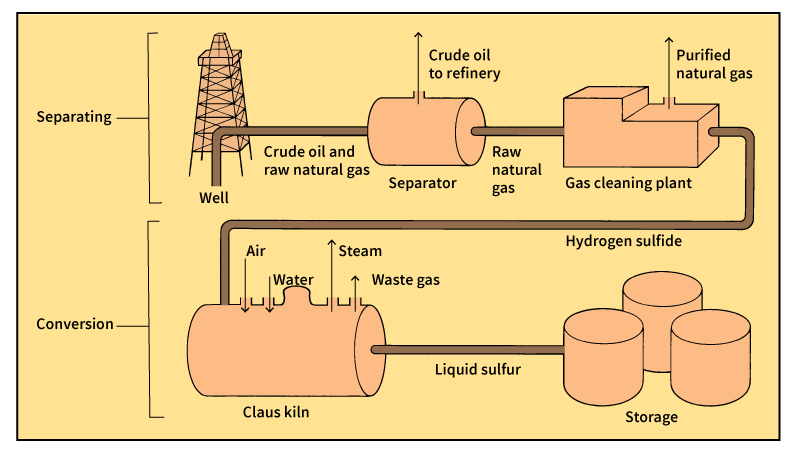
Today, most sulfur comes from sulfur compounds found in oil and natural gas. In natural gas, sulfur takes the form of hydrogen sulfide, a compound of hydrogen and sulfur. Petroleum companies extract hydrogen sulfide to prevent the release of sulfur dioxide when the natural gas is burned. After removal from natural gas, the hydrogen sulfide is heated and converted to sulfur that is 99.9 percent pure. This process, called Claus conversion, was invented in 1883 by C. F. Claus, an English chemical engineer. Sulfur is also obtained by roasting pyrites and other minerals containing sulfur to form sulfur dioxide. This gas is then used to manufacture sulfuric acid.
See also Sulfa drug ; Sulfate ; Sulfide ; Sulfur dioxide ; Sulfuric acid.
