Tellurium, << teh LUR ee uhm, >> is a rare chemical element classified as a metalloid. Pure tellurium often appears as shiny, silvery-white crystals. In nature, the element most often occurs in combination with such metals as copper, gold, lead, mercury, and silver. Pure tellurium is usually obtained as a by-product of copper refining. Tellurium is used in the production of iron, steel, and certain alloys (combinations of two or more metals). Manufacturers use tellurium in the making of semiconductors and blasting caps. Tellurium helps cure (harden) rubber and catalyzes (stimulates) chemical reactions in petroleum refining. It is also used to color glass and ceramics.
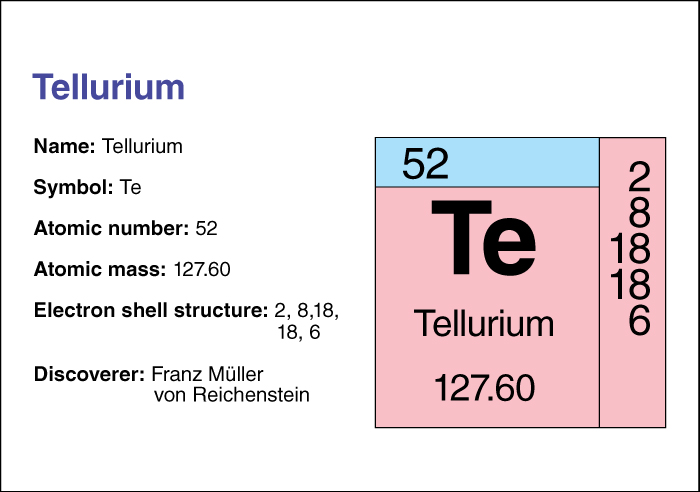
Tellurium’s chemical symbol is Te. Its atomic number (number of protons in its nucleus) is 52. Its relative atomic mass is 127.60. An element’s relative atomic mass equals its mass (amount of matter) divided by 1/12 of the mass of carbon 12, the most abundant form of carbon. Tellurium melts at 449.5 °C and boils at 989.8 °C. The Austrian chemist Franz Muller von Reichenstein discovered tellurium in 1782.
For information on the position of tellurium on the periodic table, see the article Periodic table .
