Thallium, << THAL ee uhm, >> is a soft, bluish-gray metallic element that looks like lead. Most thallium comes from iron pyrites, in which traces of the element occur as an impurity. In addition, the element occurs in the minerals crookesite, hutchinsonite, and lorandite. Sir William Crookes, an English scientist, discovered thallium in 1861.
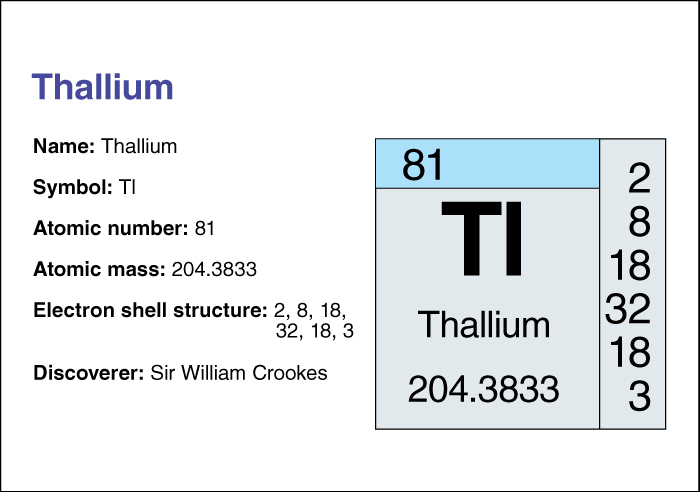
Thallium has an atomic number (number of protons in its nucleus) of 81. Its relative atomic mass is 204.3833. An element’s relative atomic mass equals its mass (amount of matter) divided by 1/12 of the mass of carbon 12, the most abundant isotope (form) of carbon. The chemical symbol of thallium is Tl. Thallium melts at 303.5 °C and boils at 1457 (plus or minus 10) °C. At 20 °C, it has a density of 11.85 grams per cubic centimeter (see Density ). For information on the position of thallium on the periodic table, see the article Periodic table .
Thallium is quite toxic to human beings. Its toxic effects are cumulative—that is, they build up over an extended period. Too much exposure to thallium may cause nerve damage, emotional change, cramps and convulsions, and eventually coma and death due to respiratory paralysis.
Thallium and its compounds have various uses. However, these uses are limited because of the chemical’s highly toxic nature. A radioactive isotope of the element, Tl-201, is useful for diagnosing certain types of heart disease. Thallium bromide, thallium iodide, and thallium sulfide undergo changes when they are exposed to infrared radiation. As a result, they are used in devices for detecting and measuring such radiant energy.
