Tin is a white metallic element that people have used since ancient times. The earliest known use of tin occurred sometime after 3500 B.C. in the Zagros Mountains of what is now western Iran. The people of that region made such articles as weapons and tools from bronze, an alloy of tin and copper. Today, tin is used chiefly in the production of tin plate. Tin plate consists of steel coated on both sides with an extremely thin film of tin. Most tin plate is made into tin cans.
Tin has the chemical symbol Sn. Its atomic number (number of protons in its nucleus) is 50. Its relative atomic mass is 118.710. An element’s relative atomic mass equals its mass (amount of matter) divided by 1/12 of the mass of carbon 12, the most abundant form of carbon. At 20 °C, tin has a density of 7.2984 grams per cubic centimeter (see Density ). The difference between its melting point, 231.9 °C, and its boiling point, 2270 °C, is one of the widest of any metal. Tin is also very malleable—that is, it can easily be formed into complex shapes. These and other properties of tin enable it to be used in the manufacture of an extremely wide variety of products.
Chemists classify tin as an other metal . For information on the position of tin on the periodic table, see the article Periodic table .
Uses.
The coating on tin cans protects the steel in the cans from rust and provides an attractive appearance. Tin also prevents the weak acids in food from damaging the inside of the cans. See Tin can.
Tin coatings also protect many other items. Most paper clips, safety pins, straight pins, and staples are made of steel or brass coated with tin. Many food-preparation containers and utensils have tin coatings.
The second most important use of tin is in solders << SOD urhz >> , which are alloys used to join metal surfaces. Solders made primarily of tin and lead are called soft solders and melt at relatively low temperatures. Other important tin alloys include bronze and pewter. See Solder ; Bronze ; Pewter.
The malleability of tin enables manufacturers to make tin into extremely thin foil. One use for such foil is as a moistureproof wrapping. Terneplate is iron coated with an alloy of lead and tin. Sheets or strips of terneplate are used for roofing and in making such products as fuel tanks and fire extinguishers.
Manufacturers improve the properties of various metals by adding small amounts of tin. For example, cast iron that contains only 0.1 percent tin is much more durable and easier to work with than ordinary cast iron. Many other products, including bearings, dental fillings, and printing alloys, also contain small amounts of tin, which improves their properties.
Tin combines with other elements to form a great number of useful compounds. Many toothpastes contain stannous fluoride, a compound of tin and fluorine that helps prevent tooth decay. Certain compounds that contain tin and carbon are used as pesticides.
Where tin is found.
Tin makes up only about 0.001 percent of Earth’s crust. As a result, the amount of tin mined annually is very small compared with other commonly used metals. Most known deposits of tin are in the Southern Hemisphere. The United States has none large enough to mine.
The principal tin ore is a compound of tin and oxygen called cassiterite. The chemical formula of this compound is SnO2. Some tin ores contain sulfur and small amounts of such other metals as copper, iron, and lead. Tin deposits sometimes occur as narrow veins that run through granite. More than 80 percent of tin ore, however, is found in plains, where flowing water has deposited bits of eroded granite and ore.
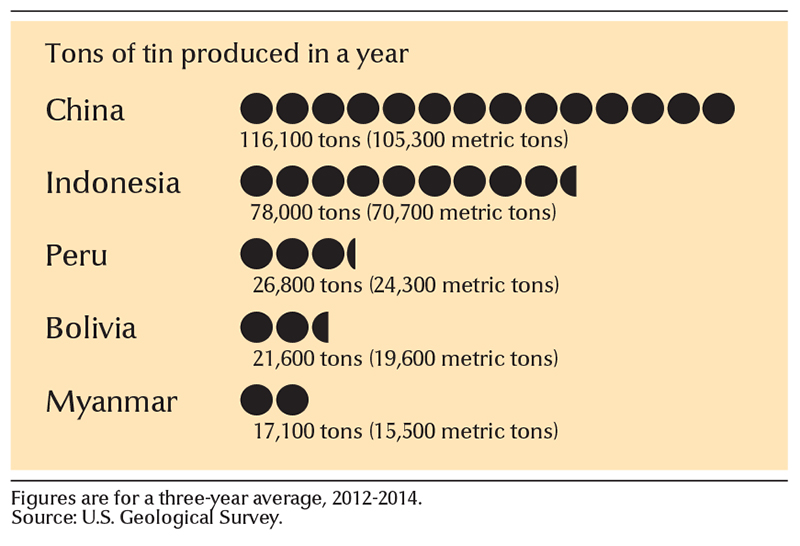
China is the world’s leading producer of tin. Other important tin-producing countries include Bolivia, Brazil, Indonesia, Myanmar, and Peru.
Refining tin.
Tin is produced by heating cassiterite with coal and limestone in a special furnace. After this process, called smelting, the tin is refined to a higher purity—usually to a purity of 99.8 percent. For details on refining, see Metallurgy (Extractive metallurgy) . Most pure tin is cast into ingots (bars) that weigh about 100 pounds (45 kilograms).
