Transmutation of elements is the transformation of an atom of one chemical element into an atom of another element. An atom’s nucleus contains one or more protons (positively charged particles). All atoms of an element have the same number of protons in their nuclei. This number is called the element’s atomic number. Transmutation occurs when the number of protons in an atom’s nucleus changes.
Transmutation commonly occurs in nature when a radioactive atom emits (gives off) a particle. This process is called radioactive decay. The most common forms of radioactive decay are alpha decay and beta decay. In alpha decay, the nucleus emits an alpha particle made up of two protons and two neutrons. The atom, with two fewer protons, becomes an atom of the element whose atomic number is two lower. For example, an atom of radium (atomic number 88) has 88 protons in its nucleus. After alpha decay, the atom has 86 protons, making it an atom of radon (atomic number 86).
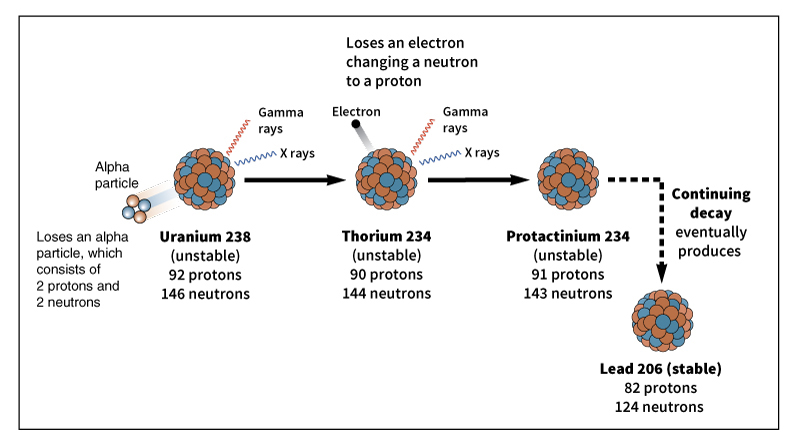
In beta decay, a neutron changes into a proton or a proton changes into a neutron. The first process emits an electron or beta particle. The atom has one more proton in its nucleus than before, changing it into an atom of the element whose atomic number is one higher. The second process emits a positively charged particle called a positron. The atom has one fewer protons in its nucleus, changing it into an atom of the element whose atomic number is one lower.
Transmutation can take place artificially when a device called a particle accelerator slams a charged particle into a nucleus. If the particle is an alpha particle, for example, the nucleus first absorbs it and then emits a proton. The atom is left with one more proton in its nucleus, making it an atom of the element whose atomic number is one higher. Artificial transmutation can also occur in a device called a nuclear reactor. Inside the reactor, neutrons can bombard the nucleus, causing it to fission (split) into two nuclei of different elements. In a fusion reactor, two nuclei fuse (merge) to form a nucleus of a different element.