Water is the most common substance on Earth’s surface. It also covers more than 70 percent of Earth’s surface area. It fills the oceans, rivers, and lakes, and is in the ground and in the air we breathe. Water is everywhere.
Without water, there can be no life. In fact, every living thing consists mostly of water. Your body is about two-thirds water. A chicken is about three-fourths water, and a pineapple is about four-fifths water. Most scientists believe that life itself began in water—in the salty water of the sea.
Ever since the world began, water has been shaping Earth. Rain hammers at the land and washes soil into rivers. The oceans pound against the shores, chiseling cliffs and carrying away land. Rivers knife through rock, carve canyons, and build up land where they empty into the sea. Glaciers plow valleys and cut down mountains.
Water helps keep Earth’s climate from getting too hot or too cold. Land absorbs and releases heat from the sun quickly. But the oceans absorb and release the sun’s heat slowly. So breezes from the oceans bring warmth to the land in winter and coolness in summer.
Throughout history, water has been people’s slave—and their master. Great civilizations have risen where water supplies were plentiful. They have fallen when these supplies failed. People have killed one another for a muddy water hole. They have worshiped rain gods and prayed for rain. Often, when rains have failed to come, crops have withered and starvation has spread across a land. Sometimes the rains have fallen too heavily and too suddenly. Then rivers have overflowed their banks, drowning large numbers of people and causing enormous destruction of property.
Today, more than ever, water is both slave and master to people. We use water in our homes for cleaning, cooking, bathing, and carrying away wastes. We use water to irrigate dry farmlands so we can grow more food. Our factories use more water than any other material. We use the water in rushing rivers and thundering waterfalls to produce electricity.
Our demand for water is constantly increasing. Every year, there are more people in the world. Factories turn out more and more products, and need more and more water. We live in a world of water. But almost all of it—about 97 percent—is in the oceans. This water is too salty to be used for drinking, farming, and manufacturing. Only about 3 percent of the world’s water is fresh (unsalty). Most of this water is not easily available to people because it is locked in ice that covers Antarctica, Greenland, and the waters of the north polar region. But there is still enough to meet people’s needs. There is as much water on Earth today as there was when dinosaurs inhabited the planet millions of years ago.
Earth holds a nearly constant amount of water and has done so for many millions of years. Almost every drop of water we use finds its way to the oceans. There, it is evaporated by the sun. It then falls back to Earth as rain. Water is used and reused over and over again. It is never used up.
Although the world as a whole has plenty of fresh water, some regions have a water shortage. Rain does not fall evenly over Earth. Some regions are always too dry, and others too wet. A region that usually gets enough rain may suddenly have a serious dry spell, and another region may be flooded with too much rain.
Some regions have a water shortage because the people have managed their supply poorly. People settle where water is plentiful—near lakes and rivers. Cities grow, and factories spring up. The cities and factories dump their wastes into the lakes and rivers, polluting them. Then the people look for new sources of water. Shortages also occur because some cities do not make full use of their supply. They have plenty of water but not enough storage tanks, treatment plants, and distribution pipes to meet the people’s needs.
As our demand for water grows and grows, we will have to make better and better use of our supply. The more we learn about water, the better we will be able to meet this challenge.
This article tells broadly about water. It discusses water’s importance to civilization and to life itself. For a discussion of our water problems and how we use and abuse our water supply, see Water pollution. Separate articles, including Climate, Conservation, Lake, Ocean, Rain, and River, provide details about the broad subject of water.
Water in our daily lives
Every plant, animal, and human being needs water to stay alive. This is because all the life processes—from taking in food to getting rid of wastes—require water. But people depend on water for more than just to stay alive. We also need it for our way of life. We need water in our homes—to brush our teeth, cook food, and wash dishes. We need water in our factories—to manufacture almost everything from automobiles to zippers. We need water for irrigation—to raise crops in regions that do not get enough rain.
Water in living things.
Every organism (living thing) consists mostly of water. The human body is usually made up of 50 to 75 percent water. A mouse is about 65 percent water. An elephant and an ear of corn are about 70 percent water. A potato and an earthworm are about 80 percent water. A tomato is about 95 percent water.
All living things need a lot of water to carry out their life processes. Plants, animals, and human beings must take in nutrients (food substances). Watery solutions help dissolve nutrients and carry them to all parts of an organism. Through chemical reactions, the organism turns nutrients into energy, or into materials it needs to grow or to repair itself. These chemical reactions can take place only in a watery solution. Finally, the organism needs water to carry away waste products.
Every living thing must keep its water supply near normal, or it will die. Human beings can live without food for several weeks, but they can live without water for only about one week. If the body loses more than 20 percent of its normal water content, a person will die painfully. Human beings should take in about 21/2 quarts (2.4 liters) of water a day. This intake can be in the form of beverages we drink, or water in food.
Water in our homes.
In our homes, we use far more water than the amount we need simply to stay alive. We require water for cleaning, cooking, bathing, and carrying away wastes. For many people, such water is a luxury. Millions of homes in Asia, Africa, and South America have no running water. The people must haul water up by hand from the village well, or carry it in jars from pools and rivers far from their homes.
The United States has more homes with kitchen faucets and flush toilets than any other country. On the average, every American uses more than 100 gallons (380 liters) of water a day in the home. It takes up to about 4 gallons (15 liters) of water to flush a toilet. It takes 20 to 30 gallons (76 to 114 liters) to take a bath, and each minute under a shower takes at least 5 gallons (19 liters). It takes more than 15 gallons (57 liters) of water to wash a day’s dishes, and about 40 gallons (152 liters) to run an automatic washing machine.
Water for irrigation.
Most of the plants that people raise need great quantities of water. For example, it takes 115 gallons (435 liters) of water to grow enough wheat to bake a loaf of bread. People raise most of their crops in areas that have plenty of rain. But to raise enough food for their needs, people must also irrigate dry areas. The rainfall that crops use to grow is not considered a water use, because the water does not come from a country’s supply. Irrigation, on the other hand, is a water use because the water is drawn from a nation’s rivers, lakes, or wells.
The water a nation uses for irrigation is important to its water supply because none of the water remains for reuse. Plants take in water through their roots. They then pass it out through their leaves into the air as a gas called water vapor. Winds carry away the vapor, and the liquid water is gone. On the other hand, nearly all the water used in our homes is returned to the water supply. The water is carried by sewer pipes to treatment plants, which return the water to rivers so it can be used again.
The United States uses about 140 billion gallons (530 billion liters) of water a day for irrigation. This is enough water to fill a lake 5 miles (8 kilometers) long, 1 mile (1.6 kilometers) wide, and 130 feet (40 meters) deep. About 40 percent of all the water used in the United States is used for irrigation. For a discussion of irrigation systems, see the article Irrigation.
Water for industry.
Industry uses water in many ways. It uses water for cleaning fruits and vegetables before canning and freezing them. It uses water as a raw material in soft drinks, canned foods, and many other products. It uses water to air-condition and clean factories. But most of the water used by industry is for cooling. For example, water cools the steam used in producing electric power from fuel. It cools the hot gases produced in refining oil, and the hot steel made by steel mills.
About 38,000 gallons (144,000 liters) of water are required to make a ton of steel or a ton of paper. Manufacturers use about 7 gallons (27 liters) of water to refine 1 gallon (3.8 liters) of gasoline. It takes about 15 gallons (57 liters) of water to brew 1 gallon of beer.
In the United States, factories and steam-producing power plants draw about 160 billion gallons (600 billion liters) of water every day from wells, rivers, or lakes. This total accounts for 48 percent of all the water used in the country. Power plants use 80 percent of this water. In addition, many factories buy water from city water systems.
Although industry uses a lot of water, only 6 percent of it is consumed. Most of the water used for cooling is piped back to the rivers or lakes from which it is taken. The water consumed by industry is the water added to soft drinks and other products, and the small amount of water that turns to vapor in the cooling processes.
Water for power.
People also use water to produce electric power to light homes and to run factories. Electric power stations burn coal or other fuel to turn water into steam. The steam supplies the energy to run machines that produce electricity. Hydroelectric power stations use the energy of falling water from waterfalls and dams to produce electricity. See Water power; Electric power.
Water for transportation and recreation.
After people learned to build crude small boats, they began using rivers and lakes to carry themselves and their goods. Later, they built larger boats and sailed the ocean in search of new lands and new trade routes. Today, people still depend on water transportation to carry such heavy and bulky products as machinery, coal, grain, and oil. See Transportation.
People build most of their recreation areas along lakes, rivers, and seas. They enjoy water sports, such as swimming, fishing, and sailing. Many people also enjoy the beauty of a quiet lake, a thundering waterfall, or a roaring surf.
Nature’s water cycle
The waters of Earth move continuously from the oceans, to the air, to the land, and back to the oceans again. The sun’s heat evaporates water from the oceans. The water rises as invisible vapor, and falls back to Earth as rain, snow, or some other form of moisture. This moisture is called precipitation. Most precipitation drops back directly into the oceans. The remainder falls on the rest of Earth. In time, this water also returns to the sea, and the cycle starts again. This unending circulation of Earth’s waters is called the water cycle or hydrologic cycle.
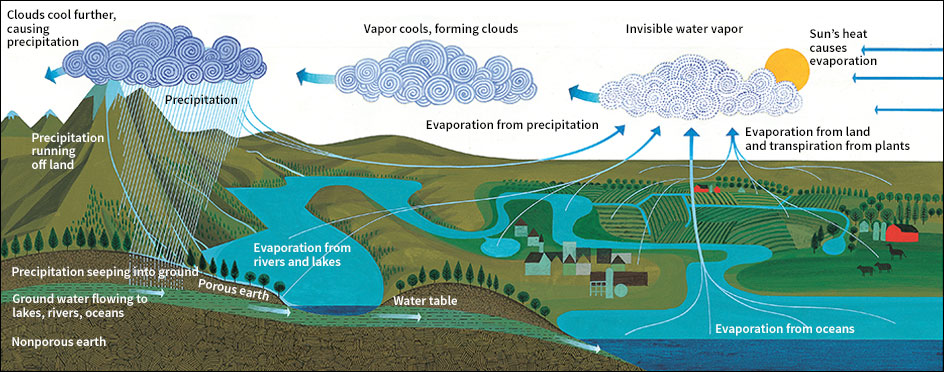
Because of nature’s water cycle, there is as much water on Earth today as there ever was—or ever will be. Water changes only from one form to another, and moves from one place to another. The water you bathed in last night might have flowed in Russia’s Volga River last year. Or perhaps Alexander the Great drank it more than 2,000 years ago.
The waters of Earth.
Earth has a tremendous amount of water, but almost all of it is in the oceans. The oceans cover about 70 percent of Earth’s surface. They contain about 97 percent of all the water on Earth, and are the source of most precipitation that falls to Earth. Ocean water is too salty to be used for drinking, agriculture, or industry. But the salt is left behind during evaporation, and the precipitation that falls to Earth is fresh water.
Only about 3 percent of the water on Earth is fresh water—and most of it is not easily available to people. It includes water locked in icecaps and other glaciers, more than 2 percent of Earth’s water. About half of 1 percent of Earth’s water is beneath Earth’s surface. Rivers and lakes contain only about one-fiftieth of 1 percent of Earth’s water.
Water in the air.
At one time or another, all the water on Earth enters the air, or atmosphere, as water vapor. This vapor becomes the life-giving rain that falls to Earth. Yet, the atmosphere contains only one-thousandth of 1 percent of Earth’s water.
Moisture in the air comes mostly from evaporation. The sun’s heat evaporates water from land, lakes, rivers, and, especially, the oceans. About 85 percent of the vapor in the air comes from the oceans. Plants also add moisture. After plants have drawn water from the ground through their roots, they pass it out through their leaves as vapor in a process called transpiration. For example, a birch tree gives off about 70 gallons (260 liters) of water a day. Corn gives off about 4,000 gallons per acre (37,000 liters per hectare) daily. See Evaporation; Leaf (Transpiration).
Precipitation.
Vapor is carried by the air moving over Earth. The moisture-filled air cools wherever it is forced up by colder air or by mountains or hills. As the air cools, the vapor condenses into droplets of liquid water, forming clouds. The droplets fall to Earth as rain. If the vapor is chilled enough, it condenses into ice crystals, and falls as snow.
About 75 percent of the precipitation falls back directly on the oceans. Some of the rest evaporates immediately—from the surface of the ground, from rooftops, from puddles in the streets. Some of it runs off the land to rivers. From the rivers, it flows back to the sea. The rest of the precipitation soaks into Earth and becomes part of the ground water supply. Ground water moves slowly through the ground to the rivers and returns to the sea. This movement of ground water to rivers keeps the rivers flowing during periods without rain. See Rain; Snow; Weather; Ground water.
How water shapes Earth.
Water changes the face of Earth as it moves through the great water cycle. Water wears down mountains, carves valleys, and cuts deep canyons. It also builds deltas and straightens coastlines.
During precipitation, some water falls on highlands and mountains. The force of gravity pulls the water downhill. As the water flows to lower levels, it erodes (wears away) the soil and rocks. In this way, after many thousands of years, mountains are worn down. The water that runs off the land during precipitation cuts small channels. The small channels drain into larger channels. The larger channels drain into still larger ones, until finally the water empties into the main stream that runs to the sea. The water then flows to the sea carrying the materials it has eroded from the land. See River.
Some of the precipitation that falls is captured in mountain glaciers. As the glaciers slide down mountainsides, they cut the mountains into sharp and jagged peaks. See Glacier.
The ocean also changes the face of the land. As waves pound against the shore, they cut away land and leave steep cliffs. Much of the material the waves wear away from the land is carried far out to sea. Some piles up near shore in sand bars. For more information on how water shapes Earth, see Earth (Cycles on and in Earth) and Erosion.
How water began.
The question of how water began on Earth is part of the question of how Earth itself began. Many scientists believe Earth was formed from materials that came from the hot sun. These materials included the elements that make up water.
As Earth cooled and grew solid, water was trapped in rocks in Earth’s crust. The water was gradually released, and the ocean basins filled with water. Other scientists have other ideas about how Earth and water began. For a discussion of these ideas, see Earth ( Early development).
The water supply problem
Shortages of fresh water have troubled people throughout history. Today, they trouble people more than ever because the demand for water is growing rapidly. Many people fear that the world does not have enough water to meet all our needs. Yet the world has—and always will have—the same amount of water it has always had. All the water we use passes through the great water cycle and can be used again and again.
The total amount of water on Earth is enough for all our needs. However, Earth’s water is distributed unevenly. Some regions suffer a constant drought (lack of rain). Other regions generally have plenty of water, but they may be struck by drought at times. In addition, people have created many water problems by mismanaging the supply.
World distribution of water.
Earth has an enormous amount of water—about 326 million cubic miles (1.4 billion cubic kilometers) of it. In a cubic mile, there are more than a million million—1,000,000,000,000—gallons, or 3.8 million million liters. However, 97 percent of this water is in the salty oceans, and more than 2 percent is in icecaps and other glaciers. The rest totals less than 1 percent. Most of this water is underground, and the remainder includes the water in lakes, rivers, springs, pools, and ponds. It also includes rain and snow, and the vapor in the air.
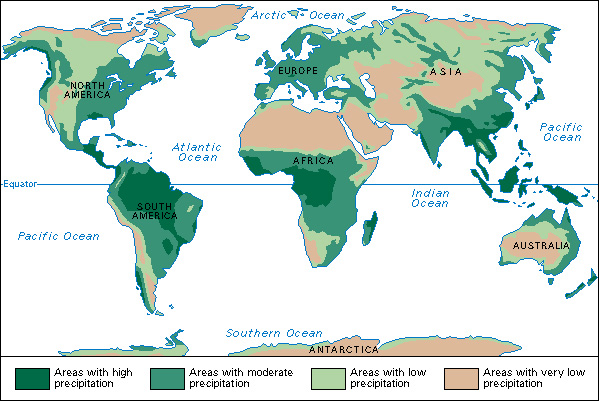
A country’s water supply is determined by its precipitation. In regions with plenty of precipitation year after year, there is plenty of water in lakes, rivers, and underground reservoirs.
Earth as a whole receives plentiful rain. If this rain fell evenly, all the land would receive 34 inches (86 centimeters) a year. But the rain is distributed unevenly. For example, over 400 inches (1,000 centimeters) drenches northeastern India every year. But northern Chile may not get rain for years.
Generally, the world’s most heavily populated areas receive enough rain for their needs. These areas include most of Europe, Southeast Asia, the Eastern United States, India, and much of China. But about half Earth’s land does not get enough rain. These dry areas include most of Asia, central Australia, most of northern Africa, and the Middle East.
The United States has plenty of water. It averages about 30 inches (76 centimeters) of rain annually. This total is large, but it is distributed unevenly. Over 135 inches (343 centimeters) soaks parts of western Washington each year, but Nevada averages only 9 inches (23 centimeters). Most states east of the Mississippi get 30 to 50 inches (76 to 130 centimeters) of precipitation a year—more than enough to grow crops. But large regions in the West get less than 10 inches (25 centimeters). There, only a small amount of grass and shrubs can grow without irrigation.
Canada’s annual precipitation is also distributed unevenly. In the southeast, it ranges from 30 inches (76 centimeters) in central Ontario to 55 inches (140 centimeters) in eastern Nova Scotia. From 14 to 20 inches (36 to 51 centimeters) of precipitation falls in most of the Prairie Provinces. Parts of the west coast get over 100 inches (250 centimeters).
Water shortages.
Many regions of the world have a constant water shortage because they never get enough rain. But even a region that normally has enough rain may suddenly have a dry year or several dry years. The climates in regions that receive only light rainfall are especially changeable. Such regions can have a series of destructive dry years.
In the 1930’s, one of the worst droughts in United States history struck the Southwest, an already dry region. Winds whipped the dry soil into gigantic dust storms, and most of the region became known as the Dust Bowl. Hundreds of farm families had to leave their homes. See Dust Bowl.
Periods of low rainfall alternate with periods of high rainfall from year to year and from place to place. In the 1980’s, for example, drought struck the Midwestern and Southeastern United States, as well as parts of Argentina, Australia, Brazil, Ethiopia, Paraguay, Uruguay, and other countries. Meanwhile, floodwaters spilled over the land in the south-central United States and in parts of Bangladesh, China, India, and other countries.
Many regions have water shortages because the people have not prepared for a period of less than normal rainfall. These shortages could have been prevented if the people had built artificial lakes, storage tanks, and other facilities to carry them through a drought.
The United States is especially rich in water. But every year, a number of U.S. communities must ration their water. As a result, many people fear that the country is running out of water. The United States as a whole has as much water today as the land had when Christopher Columbus sailed to the New World. But rainfall patterns change. In addition, the demand for water is increasing faster in the United States than in any other country. More and more Americans want air conditioners, garbage disposals, automatic washers, and an extra bathroom. Industry also demands more water as production rises. When drought strikes, the effects can be severe.
During the 1960’s, rainfall in the northeastern United States fell below normal for several years. Many cities had to restrict the use of water. New York City suffered especially, because it is so heavily populated. To save water, people turned off their air conditioners and let their lawns wither. Restaurants tried not to serve water to customers. The city was declared a disaster area. New York City’s troubles came about because the city did not have enough storage tanks, distribution lines, and other facilities to supply the city with water during a long period of light rainfall. The city improved its facilities after the drought.
Water management and conservation.
Throughout history, people have attempted to increase their water supply by trying to “make rain.” They have prayed to rain gods and performed rain dances (see Rain dance). They have sprayed the clouds with chemicals to make them release their moisture (see Cloud seeding). People also have always looked to the sea as a source of water (see the section Fresh water from the sea). In many cases, however, people do not need more water than they already have. They only need to manage the supply better.
Many water problems in the United States have arisen because the country has had a plentiful and easily available water supply. Water has been cheap, and people have been careless and wasteful. In the past, they dumped untreated sewage and other wastes into rivers and lakes, spoiling the water (see Water pollution). In most U.S. cities, people pay about $1.25 per 1,000 gallons (3,800 liters) of water. In contrast, New York City charges many people a fixed fee for water based on the size of the house or apartment building they live in. The fee is the same no matter how much water a household uses. As a result, many people waste water. However, commercial buildings and large apartment buildings in New York City have water meters, and people pay for the water they use. The city has begun to install water meters in all homes and apartment buildings.

The supply of cheap, easily available water is shrinking in the United States. The development of new supplies will become more and more costly. It will then be cheaper to reuse water from existing supplies. Many industries reuse water. For example, most steel companies use a small amount of water over and over in a circulating cooling system.
Sewage, also called wastewater, can be treated and turned into usable water. Many communities in California, Florida, Texas, and other states irrigate crops with treated wastewater. Water reuse will become much more common in the future.
City water systems
When you turn on a faucet, you expect clean, pure water to flow out. You also expect your city to have plenty of water for its industries, for fighting fires, and for cleaning streets. The job of supplying a modern city with water is tremendous. First of all, there must be sources of plentiful water to meet the demands of a growing city. Then, the water must be purified. Next, it must be piped into every house, office building, factory, and hotel in the city. Finally, the used water must be piped away.
Sources of supply.
Cities can draw fresh water from only two sources: (1) rivers and lakes, or (2) the ground. Smaller cities, especially those with fewer than 5,000 people, get water from underground supplies. Most larger cities get theirs from rivers or lakes.
Rivers and lakes.
Most cities that depend on rivers for their water are located on small rivers—simply because most rivers are small. The amount of water in a river can vary from time to time, depending on the rainfall. During a dry spell, a river’s water level may fall sharply. Then, a city may not have enough water. For this reason, many cities that depend on rivers store water during rainy periods so they will always have a good supply. Some cities build a dam on the river and store water behind it in a reservoir. Others store water in a pond or small lake. See Reservoir.
A city that draws its water from a lake has a natural storage reservoir in the lake itself. Lakes are fed by rivers and by waters moving through the ground. During wet spells, lakes store some of the extra water they receive. This extra water helps keep lake levels from dropping below normal during dry periods.
Ground water.
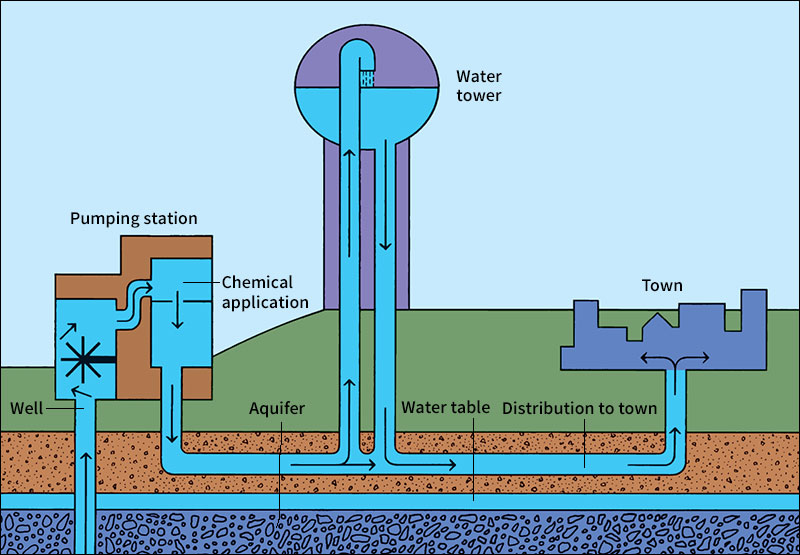
Many cities are not near rivers or lakes large enough for their needs. They use water that is stored underground. This water comes from rain that soaks into the ground. As it trickles downward, it fills the spaces between grains of sand and cracks and pores in rocks. In time, the water reaches a layer of rock or other material that is watertight. The water collects above the watertight layer, and the ground becomes saturated (soaked). This saturated zone is called an aquifer. The upper limit of the zone is called the water table. Cities obtain underground water by drilling wells that reach below the water table and pumping up the water. See Ground water; Well (Water wells).
Uses of city water supplies.
Most of the water that is supplied by public waterworks goes to people’s homes. The waterworks also deliver large amounts of water to commercial users, such as hotels and restaurants, and to manufacturers. City water systems also provide water for fighting fires, cleaning streets, and sprinkling park lawns.
Another “use” of a city’s water is waste. In many cities, homeowners pay a flat fee, no matter how much water they use. They do not have meters that measure the water they use. If their faucets leak, the water is wasted. Water is also wasted through leaks in a city’s underground pipes. Generally, water lost through leakage is 10 to 15 percent of a city’s water use. Chicago loses about 130 million gallons (490 million liters) of water daily through waste.
Purifying and treating water.
People want drinking water that is free of bacteria, sparkling clear, and without an objectionable taste or odor. Water in its natural state seldom has these qualities. So after water is drawn from a source, it is piped into a treatment plant. The plant may put the water through one or several processes, depending on the quality of the untreated water, and on a city’s standards. Many cities use three basic processes: (1) coagulation and settling, (2) filtration, and (3) disinfection.
Coagulation and settling.
The raw (untreated) water flows into the treatment plant and is mixed with chemicals. Some of these chemicals are coagulants. The most widely used coagulant is a fine powder called aluminum sulfate or alum. In the water, the alum forms tiny, sticky globs called flocs. Bacteria, mud, and other impurities stick to the flocs. The water then passes into a settling basin, where the flocs settle to the bottom. Coagulation and settling remove most impurities.
Filtration.
The water is then passed through a filter. The filter consists of a bed of sand, or of sand and coal, usually about 21/2 feet (76 centimeters) deep on top of a bed of gravel about 1 foot (30 centimeters) deep. As the water trickles down through the filter, any remaining particles are screened out. The water then flows to huge reservoirs for a final treatment that kills bacteria.
Disinfection
kills disease-carrying bacteria. Most plants disinfect water by adding a substance called chlorine. Chlorine sometimes is added before coagulation and settling, and often after filtration. Most cities chlorinate their water, even if they do not treat it in any other way. See Chlorine.
Other processes
are also used to remove unpleasant tastes or smells, or to give water special qualities. Aeration improves taste and odor. In this process, water is usually sprayed or trickled through the air. The oxygen in the air takes away the bad taste and odor. Many communities have water containing minerals that make it hard. Hard water requires lots of soap to make a lather. It also forms deposits on pipes and other equipment. Several processes can be used to soften the water (see Water softening). Some cities add lime or soda ash to their water to help prevent pipes from rusting. Activated carbon helps improve the taste and odor and remove toxic chemicals. Many communities add a substance called fluoride to their water to help reduce tooth decay (see Fluoridation).
Distributing water.
The treated water flows to a pumping station, where it is pumped into large cast iron pipes called water mains. Water mains run beneath the streets. They carry water to every fire hydrant, and connect with smaller pipes that lead to every home, office building, factory, and restaurant. The pumping station sends the water into the mains under enough pressure to carry it to every faucet. This pressure is usually so high that you cannot hold back the water by putting your finger under a fully opened faucet.
Sometimes the demand for water may be too great for the pressure a pumping station can supply. Then, water may only trickle from the faucets. This can happen on a hot summer day when many people in the neighborhood are watering their lawns or filling backyard pools. The water pressure may also fall when firefighters use a large amount of water to fight a fire.
Most cities pump water into elevated storage tanks to help keep their water pressure high at all times. The tanks are built on hills, or they are tall water towers. When water is released from these tanks, gravity pulls the water downward, giving it the pressure to rush through the water mains.

Disposing of used water.
Most of the water in our homes is used to carry away wastes. This water, and the wastes it carries, is called sewage. Factories also use water to wash away such industrial wastes as acids and greases. In most U.S. cities, a piping system under the streets carries away the sewage from homes, factories, hotels, and other buildings. This system is called a sewerage system.
Sewage has a bad odor. But more important, it contains disease-producing bacteria. Most cities have treatment plants that clean sewage water and kill the bacteria in it. The treated water can then be returned to a river, stream, or lake. To learn how sewage is treated, see the Sewage article.
Almost all of the sewage in the United States undergoes some type of sewage treatment. Only a little of the sewage is dumped untreated into rivers. The dumping of untreated sewage causes serious problems for the environment and for cities downstream that take their water from the same rivers.
Fresh water from the sea
About 97 percent of the water on Earth is in the salty oceans. In their thirst for water, people have looked longingly throughout history at this endless supply. Some brackish (slightly salty) water is found inland. Today, more than ever, many people believe that desalting ocean water and brackish water holds the answer to the ever-increasing demand for fresh water in many areas.
The salt in seawater is mostly the same substance as common table salt. A person can safely drink water that contains less than 1/2 pound of salt to 100 pounds of water, or 0.5 kilogram to every 100 kilograms. But seawater has about seven times this amount of salt. A person who drinks only seawater will eventually die. The body’s cells will dehydrate (dry out) as they try to get rid of the excess salt from the seawater. Nor can people use seawater in agriculture or industry. It kills most crops, and quickly rusts most machinery.
People have found many ways to desalinate (remove the salt from) seawater and brackish water. Desalination offers hope of relieving water shortages near the seacoasts. However, desalination does not hold the answer to all of Earth’s water problems. Even if the oceans contained fresh water, people would still have to face such problems as pollution, flood control, and water distribution.
The desalination processes used most commonly today are distillation, reverse osmosis, and electrodialysis. These processes produce fresh water from salt water.
Distillation
is the oldest method of turning salt water into fresh water. Most ocean ships use it to obtain drinking water. Seawater can be distilled simply by boiling it in a teapot, and piping the steam into a cool bottle. The steam rises, leaving the salt behind. As the steam cools in the bottle, it condenses into fresh water.
Every day, the sun evaporates millions of tons of water from the ocean’s surface. The water vapor then condenses and falls back to Earth as fresh water. For centuries, people have copied nature and used the sun’s heat to distill seawater. Two thousand years ago, Julius Caesar used solar distillation in Egypt to obtain drinking water for his soldiers.
Solar distillation can be done by filling a shallow basin with seawater and covering it with a transparent plastic dome, or a sloping sheet of glass. The salt water turns to vapor under the sun’s heat. The vapor rises until it hits the underside of the dome or glass, where it condenses. The fresh water runs down into collecting troughs. This type of distillation produces little water. In one day, such a basin in a sunny climate can produce only about a pint of water per square foot (5 liters per square meter) of the basin’s surface area. Solar distillation is not a commonly used method of distillation because it is expensive. The cost stems from the fact that this method requires the use of an enormous land area in order to produce sufficient quantities of water. Solar distillation also is less efficient in operation than other methods of distillation.
Most modern distillation plants use a process called multistage flash distillation. This is a type of the age-old method of boiling and condensation. In flash distillation, preheated seawater flows into a large chamber in which the pressure is low. The low pressure causes some of the water to flash (turn quickly) into steam. The steam is condensed into salt-free water. The seawater passes through several distillation chambers. Each of the chambers has a lower pressure than the previous chamber. Often, the final water is so pure that it is tasteless, and some salt must be tossed back in to give it flavor. The desalting plant at the United States naval base at Guantanamo Bay, Cuba, uses this process. It produces more than 1 million gallons (3.8 million liters) of fresh water a day.
Reverse osmosis
is a widely used method for desalting seawater and brackish water. In normal osmosis, a less concentrated liquid flows through a membrane into a more concentrated liquid. Thus, if salt water and fresh water are separated in a chamber by a special semipermeable membrane, the fresh water will flow through the membrane into the salt water. However, if enough pressure is placed on the salt water, this normal flow pattern can be reversed. Fresh water will then be squeezed from the salt water as it passes through the membrane, leaving the salt behind. The reverse osmosis desalting process works in this way.
A desalting plant at Cape Coral, Florida, uses reverse osmosis. This plant can produce about 14 million gallons (53 million liters) of fresh water a day.
Electrodialysis
is used chiefly to desalt brackish ground water and water from estuaries (river mouths). Electrodialysis is based on the fact that when salt is dissolved in water, it breaks up into ions (electrically charged particles) of sodium and chloride. Sodium ions carry a positive charge, and chloride ions carry a negative charge.
Electrodialysis uses a large chamber divided into many compartments by stacks of thin plastic membranes. Two types of membranes are used, and they are used in pairs. One type allows only positive ions to pass through it. The other lets only negative ions through. Two oppositely charged electric conductors, called electrodes, are placed at either end of the chamber.
When an electric current is sent through the water, the negative ions are drawn through the membranes permeable to negative ions toward one of the electrodes. The positive ions are drawn through the membranes permeable to positive ions toward the other electrode. Thus, the salt in every other compartment is drawn off, leaving fresh water.
A desalting plant on Sanibel Island, Florida, uses the electrodialysis process. This plant produces about 2 million gallons (7.6 million liters) of fresh water daily.
Other desalting processes
are also being studied. During the 1970’s, several plants experimented with freezing as a method of desalination. When seawater freezes, ice crystals of pure water form. The ice is separated, usually by washing off the salt with fresh water, and then melted into fresh, liquid water. However, high costs and engineering problems have prevented the widespread commercial use of the freezing method.
In the 1990’s and early 2000’s, some plants used the ocean’s heat energy to desalt seawater. First, they evaporated relatively warm surface water in a low-pressure chamber. They then used colder, deeper seawater to condense the vapor into fresh water in another chamber. Some researchers hoped that using the process on a large scale would cost less than standard methods.
The future of desalting.
All methods of desalination are costly, largely because desalting plants use large amounts of energy, and energy is expensive to produce. In addition, plants must pay to dispose of the salt that is removed during the desalination process. The cost of desalination depends on such factors as the capacity of the treatment plant and its location. Desalting brackish water costs less than desalting seawater. Engineers and scientists are continuing to work on the development of less expensive methods of desalination.
The thousands of desalting plants around the world together produce more than 22 billion gallons (83 billion liters) of fresh water each day. A large facility, such as the plant in Al Jubayl, Saudi Arabia, can produce about 250 million gallons (950 million liters) of fresh water daily. Although desalting plants meet only a small part of the world’s daily demand for fresh water, they are essential to millions of people.
Many desalting plants are small facilities that serve isolated military posts, oil-drilling crews in deserts, island resorts, and industrial plants. The largest number of plants are in the Middle East, where fresh water sources are scarce. As the cost for desalting water drops, more and more towns and cities may begin using desalted water.
What water is and how it behaves
Water is not only the most common substance on Earth, it is also one of the most unusual. No other substance can do all the things that water can do. Water is an exception to many of nature’s rules because of its unusual properties (qualities).
The chemistry of water.
Water consists of tiny particles called molecules. A drop of water contains many millions of molecules. Each molecule, in turn, consists of even smaller particles called atoms. Water molecules consist of atoms of hydrogen and oxygen. Hydrogen and oxygen by themselves are gases. But when two atoms of hydrogen combine with one atom of oxygen, they form the chemical compound H2O—water.
Even the purest water contains substances besides ordinary hydrogen and oxygen. For example, water contains very tiny portions of deuterium, a hydrogen atom that weighs more than the ordinary hydrogen atom. Water formed by a combination of deuterium and oxygen is called heavy water (see Heavy water; Deuterium). Water is a combination of several different substances, but these substances make up only a small fraction of it.
The properties of water.
Water can be a solid, a liquid, or a gas. No other substance appears in these three forms within Earth’s normal range of temperature. The molecules that make up water are always moving, and the form water takes depends on how fast they move. The molecules in solid water (ice) are far apart and almost motionless. The molecules in liquid water are close together and move about freely. The molecules in water vapor, a gas, move about violently and bump into one another.
Ice.
Most substances contract as they grow colder. But when water is cooled, it contracts only until its temperature reaches 39 °F (4 °C). Water expands when it becomes colder than 39 °F. For this reason, when ice forms at 32 °F (0 °C), it floats on liquid water. If water contracted upon freezing, any volume of ice would be heavier than an equal volume of liquid water. Ice would then sink. If ice sank, Earth would become a lifeless Arctic desert. Each winter, more and more ice would pile up on the bottom of lakes, rivers, and oceans. In summer, the sun’s heat could not reach deep enough to melt the ice. Water life would die. The hydrologic cycle would slow down. In time, all of the water would turn to solid ice, except perhaps for a thin layer of water over the ice during the summer.
Liquid.
Water is a liquid at temperatures found in most places on Earth. No other common substance is liquid at ordinary temperatures. In fact, the temperatures at which water is a liquid are unusual. Water is a liquid between 32 °F (0 °C), its freezing point, and 212 °F (100 °C), its boiling point. But substances with a structure like that of water are not liquid in this temperature range. These substances include gases with the formulas H2Te, H2Se, and H2S. As their formulas show, they are closely related to water (H2O). Each has two atoms of hydrogen, plus an atom of the elements tellurium, selenium, or sulfur. If water behaved like these close relatives, it would be a liquid between about –148 °F (–100 °C) and –130 °F (–90 °C). In that case, there would be no liquid water on Earth because Earth’s temperatures are far higher than –130 °F.
Water weighs about 62.4 pounds per cubic foot (1 kilogram per liter). Scientists compare the weight of other substances with the weight of water to find the specific gravity of the other substances (see Density).
Vapor.
If an uncovered glass of water stands for a few days, the water will gradually disappear because the water molecules are moving constantly. Those at the surface break free of those below and enter the air as vapor. The higher the temperature of the water, the faster it evaporates, because the water molecules move faster.
Water can also be turned into vapor by boiling it, and creating steam. It takes an enormous amount of heat to produce steam. Water boils at 212 °F (100 °C). But when water reaches the boiling point, it does not immediately turn into steam. First there is a pause, during which the water absorbs additional heat without any rise in the temperature. This heat is called latent heat. More than five times as much heat is required to turn boiling water into steam as to bring freezing water to a boil. Thus, steam holds a great amount of latent heat energy. People use this energy to run machinery.
Water vapor in the air also holds a tremendous amount of latent heat energy. This energy is released when the vapor cools and condenses, and falls as rain. The high latent heat of water is related to water’s remarkable heat capacity.
Heat capacity
is the ability of a substance to absorb heat without becoming much warmer itself. Water has a greater heat capacity than any other substance except ammonia. To illustrate water’s unusual heat capacity, imagine a pound of water, a pound of gold, and a pound of iron—all at –459.67 °F (–273.15 °C). This is absolute zero, the temperature at which a substance supposedly contains no heat at all. If all three substances were heated and each absorbed the same amount of energy, the gold would melt at 2016 °F (1102 °C). But the ice would still be at –300 °F (–184 °C). When the iron began to melt at 2370 °F (1299 °C), the ice would finally have reached 32 °F (0 °C).
Surface tension
is the ability of a substance to stick to itself and pull itself together. Water’s surface tension is extremely high. A dripping faucet shows how water sticks to itself. As the water drips, each drop clings to the faucet, stretches, lets go, and then snaps into a tiny ball. Water molecules cling together so tightly that water can support objects heavier than itself. For example, a needle or a razor blade can float on water. Insects can walk on water. Water can also stick to other substances, such as cloth, glass, and soil. By sticking to these substances, water wets them. See Surface tension.
Capillary action
is the ability of a liquid to climb up a surface against the pull of gravity. You can see water’s climbing ability in a glass of water. The water is higher around the edges, where it touches the glass. The capillary action of water helps it circulate through soil, and up through the roots and stems of plants. It also helps circulate blood, which is mostly water, throughout our bodies. See Capillary action.
Dissolving ability.
Water can dissolve almost any substance. It dissolves the hardest rocks as it runs over the land and seeps through the ground. In time, it carries the dissolved materials to the oceans. Water also dissolves the nutrients that all living things need. Water dissolves and carries the nutrients in the soil to plants and to the cells within plants. Water also dissolves the food that people and animals eat, and then carries this food to the cells.
How water is held together.
Water’s unusual properties depend on the forces that hold it together. These forces are (1) chemical bonds and (2) hydrogen bonds.
Chemical bonds
are the forces that hold the two hydrogen atoms and the one oxygen atom together in a water molecule. Each hydrogen atom has one electron whirling in orbit around its nucleus. But each of these atoms has room for two electrons. The oxygen atom has six electrons in its outer orbit, but it has room for eight. The hydrogen and oxygen atoms each fill their empty spaces by sharing their electrons. The two electrons from the two hydrogen atoms enter the orbit of the oxygen atom. At the same time, two electrons from the oxygen atom fill the empty spaces in the two hydrogen atoms. The resulting water molecule is an extremely tight structure.
Hydrogen bonds
are the forces that link water molecules together. Water molecules have a lopsided shape because the two hydrogen atoms bulge from one end of the oxygen atom. The hydrogen end of the water molecule has a positive electric charge. At the opposite end, the molecule has a negative charge. Water molecules link together because the positive and negative charges attract. The positive ends of water molecules attach to the negative ends of other water molecules, whose positive ends attach to the negative ends of still other water molecules.
Water and the course of history
Water and civilization.
Water has been vital to the development and survival of civilization. The first great civilizations arose in the valleys of great rivers—in the Nile Valley of Egypt, the Tigris-Euphrates Valley of Mesopotamia, the Indus Valley of Pakistan and northwestern India, and the Huang He Valley of China. All these civilizations built large irrigation systems, made the land productive, and prospered.
Civilizations crumbled when water supplies failed or were poorly managed. Many historians believe the Sumerian civilization of ancient Mesopotamia fell because of poor irrigation practices. Salt in irrigation water is left behind during evaporation and tends to build up in the soil. The accumulation can be avoided by washing the salt away with extra water. But if the land is not well drained, it becomes water-logged.
The Sumerians failed to achieve a balance between salt accumulation and drainage. As a result, the salt and excess water harmed their crops. Farm production gradually declined, and food shortages developed. With the collapse of agriculture, the Sumerian civilization fell.
The challenge of today,
as in ancient times, is for people to make the best use of water. But the challenge is greater than ever before because more water is needed as the world’s population increases. Scientists estimate that nearly 50 countries will face water shortages by 2025. Also, many people do not conserve water, and they pollute water and manage it poorly in other ways.
Countries are working together to try to solve water problems. The United Nations (UN) has been heavily involved in these efforts. In addition, groups of countries whose lands are drained by major rivers and seas have formed regional organizations to fight water pollution.
From 1965 through 1974, about 70 countries took part in the International Hydrological Decade, a UN program established to promote scientific research on water resources. In 1975, the UN founded the International Hydrological Programme (IHP) to continue the research. The IHP is a long-term program that is carried out in phases lasting three or more years. Each phase has a theme. For example, the theme of the fifth phase, for the years 1996 to 2001, is “Hydrology and Water Resources Development in a Vulnerable Environment.”
In 1993, the UN General Assembly declared March 22 of each year as World Day for Water. Each year, the day’s activities promote public awareness of issues related to the protection and use of fresh water.
One regional antipollution group is the International Commission for the Protection of the Danube River, which was established in 1998. Members of the commission include 12 nations whose lands are drained by the Danube in Europe. The organization operates an early warning network for oil spills and other pollution alerts. One of the commission’s main projects is to recommend plans for cleaning up the sources of the most severe pollution of the Danube.
