Ozone hole is a region of the atmosphere over Antarctica where a protective layer of ozone gas, a form of oxygen, becomes less concentrated every spring. The ozone layer normally blocks the sun’s harmful ultraviolet rays, protecting life on Earth.
Most of Earth’s ozone occurs in a layer 10 miles (16 kilometers) thick that begins 10 miles above Earth’s surface and reaches to 20 miles (32 kilometers) above the surface. Over the North and South poles, the ozone layer extends from 6 to 20 miles (10 to 32 kilometers) above the surface. Scientists say that the ozone layer is depleted when ozone molecules in this layer become less concentrated.
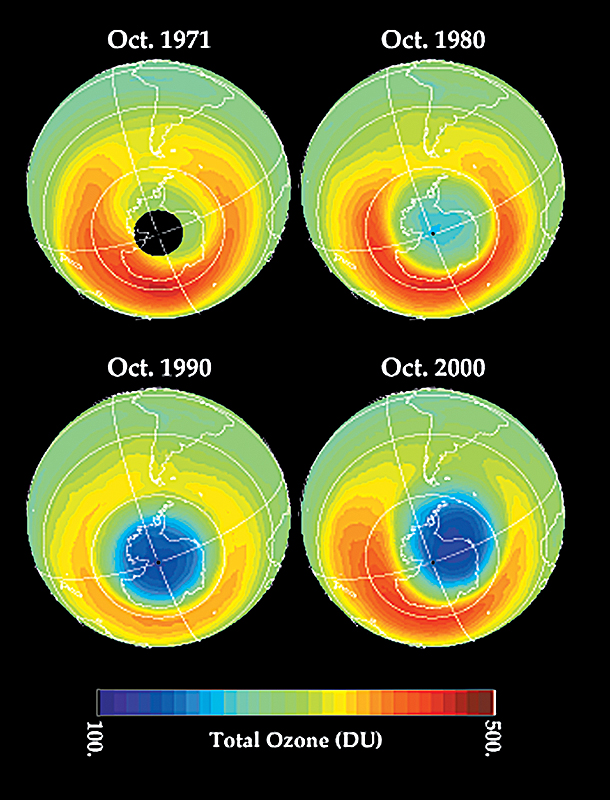
Scientists began monitoring ozone continuously over Antarctica in 1957 during the International Geophysical Year, a period of international cooperation in scientific research. In 1985, researchers led by the British atmospheric scientist Joseph C. Farman showed that the ozone hole had first appeared in the 1970’s. Ozone concentrations over Antarctica weakened and the area of low concentrations grew larger nearly every year thereafter until the end of the 1900’s.
An ozone hole is created by a combination of cold weather and chemical activity. The chemical activity begins when certain compounds rise to the upper atmosphere near Earth’s equator. The most important compounds in this regard are chlorofluorocarbons (CFC’s). In the air, these compounds are broken down by ultraviolet rays and release chlorine atoms. The process also releases some bromine atoms. The air is then carried toward Antarctica by winds in Earth’s upper atmosphere. Because Antarctica receives little or no sunshine from April through August, the air above it becomes extremely cold. Eventually, a strong wind known as the polar jet prevents this cold air from mixing with warm air located to the north. As air in the upper atmosphere continues to cool over the Antarctic winter, water vapor and other gaseous compounds condense into small ice particles that form polar stratospheric clouds (PSC’s).
The ozone hole occurs each year between August and October, during which the amount of ozone over Antarctica is depleted by about two-thirds. In late August, when sunlight returns to Antarctica, certain chemical reactions rapidly begin taking place on ice particles in PSC’s. These reactions convert certain forms of chlorine into molecules that destroy ozone. By the end of November—late spring in Antarctica—the air over Antarctica becomes warmer, stopping PSC’s from forming and halting the reactions that deplete ozone. As the polar jet weakens, warm, ozone-rich air from the north replenishes the ozone layer over Antarctica.
Ozone depletion also occurs over the Arctic. However, depletion there is usually less severe because Arctic air is not as cold as Antarctic air.
CFC’s are industrially produced substances. They were once widely used as refrigerants and as propellants in aerosol spray cans. By international agreement, most countries have halted the production of CFC’s. However, CFC’s already released into the atmosphere will contribute to formation of the ozone hole for many decades.
In 2016, scientists announced that the ozone hole was showing signs of healing as CFC’s were slowly removed from the atmosphere by natural processes. Between the years 2050 and 2100, the ozone hole is expected to decrease to a size not seen since 1980. However, this recovery is expected only if the international ban on CFC’s remains in force. Unfortunately, in 2019, routine monitoring of the atmosphere revealed unexplained emissions of one potent CFC, CFC-11. If not corrected, these unexplained emissions would delay the healing of the ozone hole.
