Metalloid, also called a semimetal, is a chemical element with some properties of a metal and some properties of a nonmetal. The metalloids are found on the right side of the periodic table, where the metals and nonmetals meet. Many periodic tables use a dark, zigzag line to separate the metals and nonmetals. The metalloids all border this line, but chemists disagree on which bordering elements should be called metalloids. Many chemists consider the metalloids to be antimony, arsenic, boron, germanium, polonium, silicon, and tellurium. Carbon is considered a metalloid in its graphite form but a nonmetal in its diamond form. Other nonmetals, such as phosphorus and selenium, exhibit metalloid characteristics in some forms.
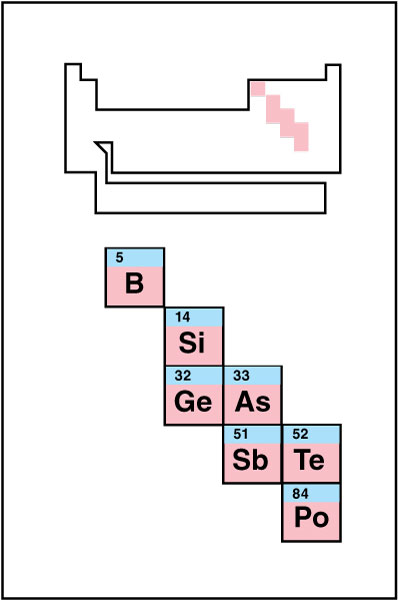
Metalloids have metallic and nonmetallic properties. For example, the element silicon looks silvery and shiny in its pure form, much like a metal. However, silicon does not conduct heat or electric current well, a characteristic typical of nonmetals. Also, elemental carbon is black and nonconductive, unlike metals, but graphite is gray and shiny, and conducts current well, like a metal.
Many metalloids change their properties as impurities are added to them, a useful characteristic. For example, adding certain other elements to silicon can increase its electrical conductivity. Manufacturers widely use silicon semiconductors made in this way in computers and electronic equipment.
Metalloids have other industrial uses. Antimony is used in some kinds of matches, and it can be added to other metals to form alloys (mixtures). Arsenic is used in some pesticides. Boron is used in nuclear reactors, and to make heat-resistant glass.
