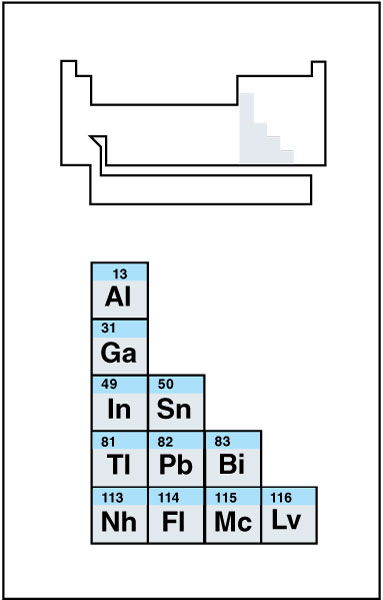
Other metal is a term used to describe those metallic elements in the periodic table that do not belong to any other grouping, such as the transition metals or alkaline earth metals. People sometimes refer to the other metals as poor metals or simply metals. The other metals appear on the right side of the periodic table, between the transition metals and the metalloids. The other metals usually include aluminum, bismuth, gallium, indium, lead, thallium, and tin. Chemists expect the artificially produced radioactive elements nihonium (Nh), flerovium (Fl), moscovium (Mc), and livermorium (Lv) to be other metals.
The other metals have lower melting points and boiling points than most of the transition metals. For example, gallium melts at 85.6 °F (29.8 °C), lower than human body temperature. The other metals are also softer than most transition metals. For example, people can bend sheets of lead by hand.
Aluminum ranks as the second most widely produced metal, after iron. It resists corrosion better than many iron alloys (mixtures of metals). Aluminum alloys can be both strong and lightweight at the same time. People use aluminum in the construction of cars, planes, buildings, packaging materials, electrical wire, cookware, and gardening tools. Lead has few remaining uses because it is toxic. But manufacturers still use lead in several ways, such as in storage batteries for cars, boats, and planes.
Food packagers use both aluminum and tin for canning. Many toothpastes contain a tin compound called stannous fluoride, which helps prevent tooth decay. Tin also serves as an important component of the alloys pewter and bronze and some solders (alloys used to join metal surfaces). Manufacturers use gallium and indium to make electronic equipment. Toxic thallium has limited uses. Bismuth is often mixed with other metals to form alloys that melt at low temperatures. It also has uses in medicines, foundries, and nuclear reactors.
