Transition metal is any of a series of metallic chemical elements found in the center of the periodic table. These elements form a connection or transition between the alkali metals and what are known as the other metals. The transition metals include many commonly recognized metals, such as copper, gold, iron, nickel, and silver.
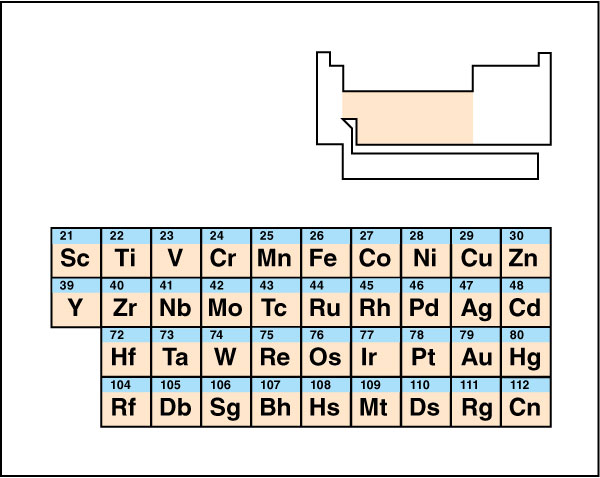
Traditionally, the transition metals occupy the 4th, 5th, 6th and 7th periods (rows) of the periodic table in groups (columns) 3 through 12. This area of the table is known as the d block. Proceeding left to right through the d block, electrons are usually being added to a region of the atom called a d subshell. For example, an atom of scandium has one electron in its 3rd subshell, while the next element, titanium, has two.
Alternatively, scientists sometimes define a transition metal as any element whose atoms include a partially filled d subshell. This definition excludes cadmium, mercury, and zinc, whose d blocks are completely filled. Another definition excludes elements whose ions (electrically charged atoms) do not have partially filled d subshells. This would also exclude scandium and yttrium from the list. These alternate definitions are useful because partially filled d subshells give the transition metals many of their characteristic properties.
Transition metals vary in their chemistry. Many of them can form more than one stable positive ion. This fact enables them to form a wide variety of compounds.
Some transition metals serve as important components of proteins, enzymes, and other biological substances. These elements include cobalt, iron, nickel, and vanadium.
Iron ranks as the most widely used transition metal, accounting for more than 90 percent of all metal produced industrially. Many transition metals have brightly colored compounds used in dyes, paints, and other colorings.
The elements of atomic number 104 (rutherfordium) to 112 (copernicium) are generally classified as transition metals, but are highly radioactive.
