Carbon is one of the most familiar and important chemical elements. All living things are based on carbon. Industry uses it in a wide variety of products. Yet carbon makes up only 0.032 percent of Earth’s crust. Carbon is the main component of such fuels as coal, petroleum, and natural gas. Carbon is also found in most plastics, many of which are derived from carbon fuels.
Chemical properties
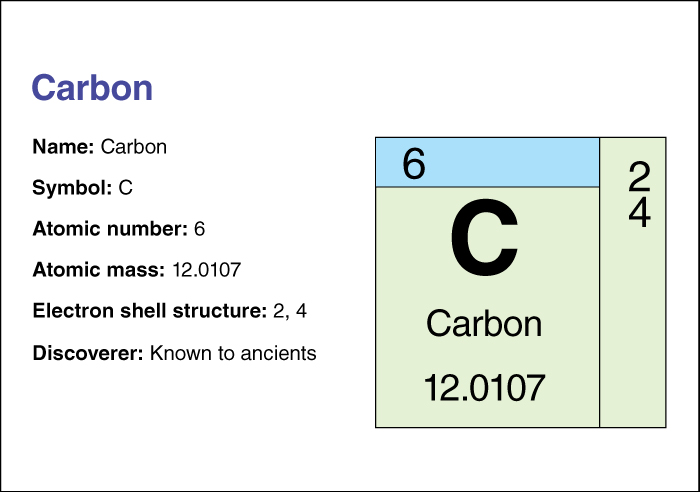
Carbon has the chemical symbol C. Pure carbon does not react readily with other chemicals at room temperature. Most naturally occurring forms of carbon, such as diamond and graphite, do not dissolve in acid or any other common solvent. Carbon solids are stable up to high temperatures in the absence of oxygen. At reduced pressures, some forms of carbon sublime (change from a solid to a vapor without melting).
Carbon’s atomic number (number of protons in its nucleus) is 6. The most abundant isotope of carbon is carbon 12. The isotopes of an element have the same number of protons but different numbers of neutrons.
Carbon 12 is the international standard for a measurement known as the relative atomic mass of an isotope or an element. By agreement, carbon 12 has a mass (amount of matter) of exactly 12 unified atomic mass units (u). The mass of any other isotope or element is expressed in terms of these units. For example, the mass of copper is expressed as 63.55 u. An element’s relative atomic mass is represented by the same number that represents its mass in unified atomic mass units. But the relative atomic mass does not include the “u”. Thus, the relative atomic mass of copper is 63.55. The relative atomic mass of an element with two or more natural isotopes is an average value based on the proportions in which the isotopes occur in nature. For this reason, the relative atomic mass of carbon is not precisely 12, but 12.0107.
Chemists classify carbon as a nonmetal. For information on the position of carbon on the periodic table, see the article Periodic table.
Carbon atoms have unusual ability to combine with other atoms. They can form strong chemical bonds with two, three, or four other atoms. These atoms can be carbon atoms or atoms of other chemical elements. Carbon atoms can link together to form long chains, rings, or combinations of chains and rings. This unique linking ability enables carbon to form the complex molecules that make up living things. Carbon atoms also combine to form balls and tubes.
Carbon compounds
Much of the carbon on Earth exists in combination with other elements. There are more than 1 million known carbon compounds. That ranks as the largest number of compounds formed by any element except hydrogen. One of the most abundant carbon compounds is the gas carbon dioxide, which is part of the atmosphere. Other plentiful compounds are the carbonate minerals, such as limestone (also known as calcium carbonate) and marble. Also abundant are the hydrocarbons. Hydrocarbons are compounds of carbon and hydrogen that are the chief ingredients of the fuels petroleum and natural gas.
Carbon compounds make up the living tissues of all plants and animals. Organic chemistry—the study of compounds made by and derived from living things—is primarily the study of carbon compounds. Most organic compounds consist mainly of carbon combined with hydrogen, nitrogen, and oxygen in various proportions.
Forms of carbon
Pure carbon occurs in four forms—diamond, graphite, amorphous carbons, and fullerenes. The four forms have different crystalline structures—that is, their atoms are arranged differently. The various forms of carbon differ greatly in hardness and other properties, depending on how their atoms are arranged.
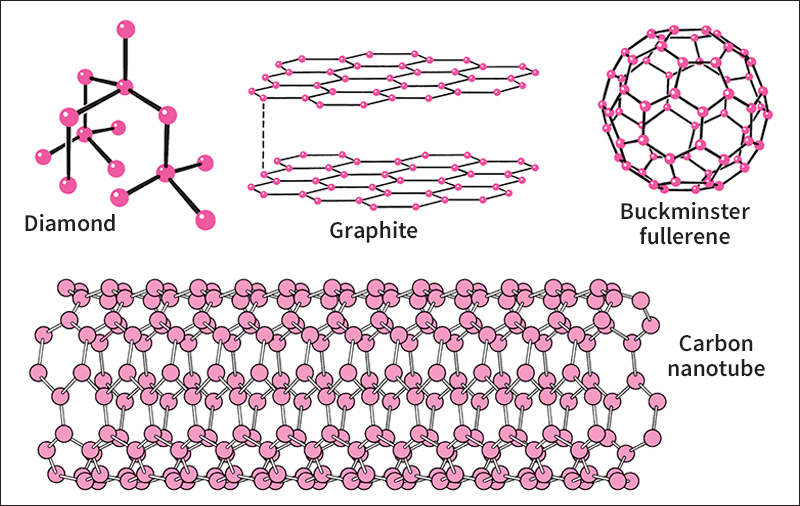
Diamond
is the hardest naturally occurring substance. It is also one of the most valuable. Natural diamonds form in the rock beneath Earth’s crust. There, high temperature and pressure cause carbon atoms to make strong bonds with four other carbon atoms each and to crystallize. Volcanic activity then forces the diamonds to the surface. Manufacturers produce artificial diamonds by heating and compressing pure carbon, usually graphite. Scientists grow synthetic diamond coatings by placing the object to be coated in a special chamber. In the chamber, a carbon-rich gas separates chemically and deposits a carbon film on the surface of the object.
The atoms in a diamond are arranged in a pyramid-shaped pattern called a tetrahedron. This arrangement of atoms makes the structure extremely rigid. The density of diamond is 3.5 grams per cubic centimeter.
Only a small percentage of natural diamonds are pure and perfect enough to become gemstones. Most diamonds, whether natural or synthetic, are used for industrial purposes. Because of diamonds’ hardness, manufacturers use them to shape, cut, grind, and polish hard materials. Diamonds have another unique pair of properties—they are good conductors of heat but do not conduct electrical current. Diamond films are thus used in high-power electronic devices to remove excess heat without affecting the device’s electrical characteristics.
The crystalline structure of diamond is the same as that of silicon, the chief material used in transistors. As a result, transistors can also be made from diamond. Diamond transistors can be safely used under much harsher conditions, such as extremely high temperatures, than ordinary silicon transistors can.
Graphite
is a soft, steel-gray or black mineral. It feels slick to the touch. Like diamond, natural graphite forms beneath the surface of Earth. Perfect graphite crystals are rare and hard to find. Low-grade graphite, however, is plentiful. Industry produces synthetic graphite by heating coke, a solid fuel that contains about 90 percent carbon.

Graphite consists of carbon atoms arranged in flat, parallel layers. A single sheet is called graphene. The layers slide easily over one another, making the graphite soft and slippery. Graphite is much less dense than diamond. It has a density of only 2.2 grams per cubic centimeter.
Because graphite is slick and soft, it is used in powdered lubricants and for the “lead” in some pencils. Unlike diamond, graphite is a good electrical conductor. As a result, it is used to make the contacts in electric motors and other machinery. Because graphite fibers are strong, they are used to reinforce plastic. Graphite and plastic form a strong, lightweight composite material. The composite is used to make dish antennas, tennis rackets, fishing rods, bicycle frames, and spacecraft parts.
Amorphous carbons,
also called glassy carbons, are made of tiny, irregularly arranged particles of graphite. The particles have no regular crystalline structure. Familiar amorphous carbons include the fuels charcoal and coke.
Amorphous carbons form, along with ash, when carbon-rich substances are heated or burned in an airtight furnace. In an airtight furnace, the burning substances lack enough oxygen to convert all the carbon to carbon dioxide. Charcoal, for example, is obtained by burning wood in the absence of air. A powdery soot called carbon black forms when natural gas or a petroleum-based fuel, such as kerosene, is burned in the same way. Carbon black is used as the black pigment in automobile tires and printing inks. A similar process using coal or petroleum produces coke and a tarry residue called pitch. Coke is an essential raw material in converting iron to steel.

Amorphous carbons have a wide range of properties. They have low densities and are porous. Carbon aerogels, also called “frozen smoke,” are among the world’s lightest solids. They have densities as low as 0.04 gram per cubic centimeter. The plentiful pores in charcoal trap many substances effectively. For this reason, charcoal is used to filter impurities from liquids and the air. The pores also enable oxygen to penetrate rapidly inside the charcoal, making it a good fuel. Amorphous carbons are also hard. They are resistant to high temperatures. In addition, they are chemically inert—that is, they do not react with most other chemicals. Because of their heat resistance, they are used for shields to protect missiles and spacecraft from getting too hot when they reenter Earth’s atmosphere.
Fullerenes
are hollow molecules made up of a large, even number of carbon atoms, 32 or more. The best known of these molecules are buckminsterfullerenes, also known as C60‘s or buckyballs. Each buckminsterfullerene consists of 60 carbon atoms bonded together in the shape of a soccer ball. Small amounts of fullerenes occur naturally in rock and in sooty flames, such as those of candles. They have been detected floating close to old stars and in the space between stars. Scientists also make fullerenes in the laboratory.
A fullerene with 70 atoms is shaped somewhat like a rugby ball. Fullerenes with more than 70 atoms can be ball-shaped or tubular. The tubes can have open or closed ends. Tubular fullerenes are sometimes called buckytubes or carbon nanotubes.
Fullerenes were first produced in 1985 by chemists Harold W. Kroto of the United Kingdom, Richard E. Smalley and Robert F. Curl, Jr., of the United States, and two of Smalley’s students. Kroto, Smalley, and Curl won the 1996 Nobel Prize in chemistry for their contributions to the discovery. The scientists vaporized graphite with a laser, producing clusters of 60 and 70 carbon atoms each. They named the C60 molecule buckminsterfullerene because its structure resembles a geodesic dome. The geodesic dome was a type of structure designed by American engineer R. Buckminster Fuller. The chemists named the entire group of hollow carbon molecules fullerenes.
It became much easier to study fullerenes in 1990. In that year, physicists Donald R. Huffman of the United States and Wolfgang Kratschmer of Germany devised a simple method for large-scale production of the molecules. As Kroto and his colleagues had done, Huffman and Kratschmer generated fullerenes by vaporizing graphite. They did it, however, by setting up two graphite rods with their ends almost touching, Then they sent an electric current across the gap. This process generated a sooty material containing about 10 percent fullerenes. The scientists extracted and purified the fullerenes with the solvent benzene.
Buckytubes and ball-shaped fullerenes have a number of properties that may prove to be of commercial value. Filled with metal atoms, for example, buckytubes form the smallest wire imaginable. The buckyball (C60) can be chemically modified to block a key step in the reproduction of the human immunodeficiency virus (HIV), which causes AIDS. Fullerenes can also be made into superconductors. Superconductors are substances that conduct electric current with no resistance at extremely low temperatures.
