Moscovium << MAHS koh vee uhm >> is an artificially produced radioactive chemical element. It has the chemical symbol Mc and anatomic number (number of protons) of 115. Chemists place moscovium in the transactinide group of transuranium elements. For information on the position of moscovium on the periodic table, see the article Periodic table.
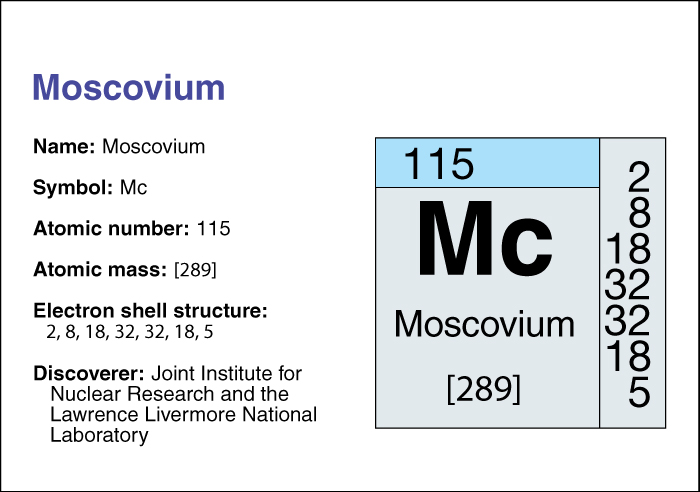
In 2003, the creation of four atoms of element 115 was announced by Russian and American physicists and chemists working at the Joint Institute for Nuclear Research in Dubna (near Moscow), Russia. In 2015, the International Union of Pure and Applied Chemistry (IUPAC) assigned credit for the discovery of the element to the Dubna team and to collaborating teams at the Lawrence Livermore National Laboratory in Livermore, California, and the Oak Ridge National Laboratory in Oak Ridge, Tennessee. IUPAC is the recognized authority in crediting the discovery of elements and assigning names to them. Element 115 was officially named moscovium in 2016. It is named in honor of the Moscow region, where the Joint Institute is located.
The researchers at Dubna produced two isotopes of moscovium. Isotopes are forms of a chemical element with the same number of protons but different numbers of neutrons. The atomic mass numbers (total numbers of protons and neutrons) of those isotopes were 287 and 288. The scientists created moscovium in a device known as a particle accelerator They bombarded the element americium, which has an atomic number of 95, with calcium, atomic number 20.
In 2010, the researchers created a more stable isotope of moscovium, number 289, while working to synthesize element 117 (now called tennessine). The researchers estimated that the half-life of isotope 289 is 0.22 second—that is, due to radioactive decay, only half the atoms in a sample of isotope 289 would still be atoms of that isotope after 0.22 second. To determine an isotope’s half-life with much accuracy, scientists must study many atoms of the isotope. When only a few atoms have been detected, as in the case of isotope 289, scientists can obtain only an approximate value of the half-life.
In the periodic table of the elements, moscovium is included with Group 15 (5A), a family of elements that includes nitrogen and phosphorus. Elements in the same family group have related properties because they have the same arrangement of electrons in their outermost or valence shell. Scientists can predict some of the properties of moscovium from its position in the periodic table. However, it is not possible to confirm some of these predictions without obtaining many more atoms of the element.
