Cerium << SIHR ee uhm >> is a soft, gray metal of the lanthanide group of chemical elements. Cerium was discovered in 1803 by the Swedish chemist Jons Berzelius and the Swedish geologist Wilhelm von Hisinger, and independently by the German chemist Martin Klaproth. It is named for Ceres, which is a large asteroid and a dwarf planet.
Cerium is the most abundant of the lanthanide elements. It is found in many minerals and is obtained commercially from the minerals monazite and bastnasite. Radioactive isotopes (forms) of cerium occur during the fission (nuclear splitting) of uranium, thorium, and plutonium.
Cerium differs from the other lanthanide elements in the ease with which its electron structure may be changed. It is added to alloys to strengthen them. It is also used to remove fission products from melted uranium. Cerium oxide is used in making porcelain and in polishing glass.
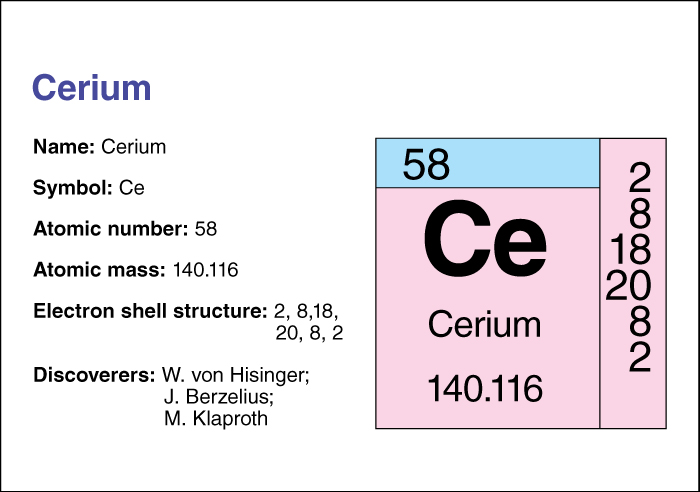
The chemical symbol for cerium is Ce. Its atomic number (number of protons in its nucleus) is 58. Its relative atomic mass is 140.116. An element’s relative atomic mass equals its mass (amount of matter) divided by 1/12 of the mass of carbon 12, the most abundant isotope of carbon. Cerium has a melting point of 798 °C and a boiling point of 3433 °C. It has a density of 6.773 grams per cubic centimeter at 25 °C. For information on the position of cerium on the periodic table, see the article Periodic table.
See also Berzelius, Jons Jakob; Element, Chemical; Rare earth.
