Cesium, << SEE zee uhm, >> is a soft, silvery metallic element. Dissolved cesium salts, such as cesium carbonate and cesium chloride, are widely distributed in low concentrations in brines and mineral waters. The German scientists Robert Bunsen and Gustav Kirchhoff first detected cesium in 1860. In 1882, the chemist Carl Setterberg isolated the pure metal.
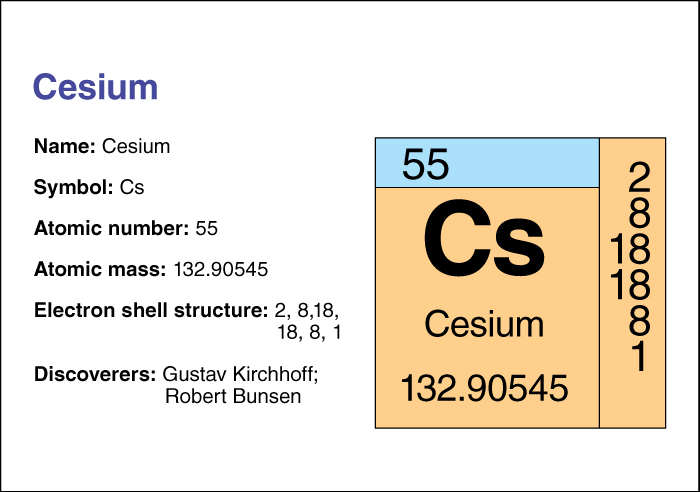
Cesium has an atomic number (number of protons in its nucleus) of 55. Its relative atomic mass is 132.90545. An element’s relative atomic mass equals its mass (amount of matter) divided by 1/12 of the mass of an atom of carbon 12, the most abundant form of carbon. Cesium’s chemical symbol is Cs. It reacts vigorously with air and water. Cesium has a melting point of 28.6 °C and a boiling point of 670 °C. At 20 °C, it has a density of 1.873 grams per cubic centimeter (see Density ). Chemists classify cesium as an alkali metal . For information on the position of cesium on the periodic table, see the article Periodic table .
Element, Chemical (table: Table of the elements)
Most cesium metal is obtained from cesium chloride through a special chemical process. Cesium ionizes readily when heated or struck by light. Because of this property, it is used in photomultiplier tubes that measure weak light (see Photomultiplier tube ). Scientists are studying the use of cesium as a fuel in ion-propulsion engines for space vehicles. They also are experimenting with methods of power generation that involve the ionization of cesium.
