Chemistry is the scientific study of substances. Chemists investigate the properties (characteristics) of the substances that make up the universe. They study how those substances behave under different conditions. They attempt to explain the behavior of a substance in terms of the substance’s structure and composition. Chemists also seek to understand chemical changes. Chemical changes involve alterations in a substance’s chemical makeup. The combination of iron with oxygen from the air to form rust is a chemical change. Substances may also go through physical change without altering their chemical makeup. Water changes physically but not chemically when it freezes.
Chemical changes occur constantly in nature. Such changes make life on Earth possible. During a thunderstorm, for instance, lightning causes a chemical change in the air. The electrical energy and heat of a lightning bolt cause some of the nitrogen and oxygen in the atmosphere to combine. The nitrogen and oxygen form gases called nitrogen oxides. The nitrogen oxides dissolve in raindrops that fall to the ground. In the soil, they are chemically changed into nitrates, substances that serve as fertilizer.
Chemical changes also occur as wood burns and becomes ashes and gases. The food we eat goes through many chemical changes in our bodies.
Chemists have learned much about the chemical substances and processes that occur in nature. In addition, chemical researchers have created many useful substances that do not occur naturally. Products resulting from chemical research include many artificial fibers, drugs, dyes, fertilizers, and plastics. The knowledge gained by chemists and the materials they have produced have greatly improved people’s lives.
The work of chemists
Chemistry involves the study of many substances. Substances differ in properties, structure, and composition. The methods chemists use and the questions they attempt to answer also differ. However, all chemists share certain fundamental ideas.
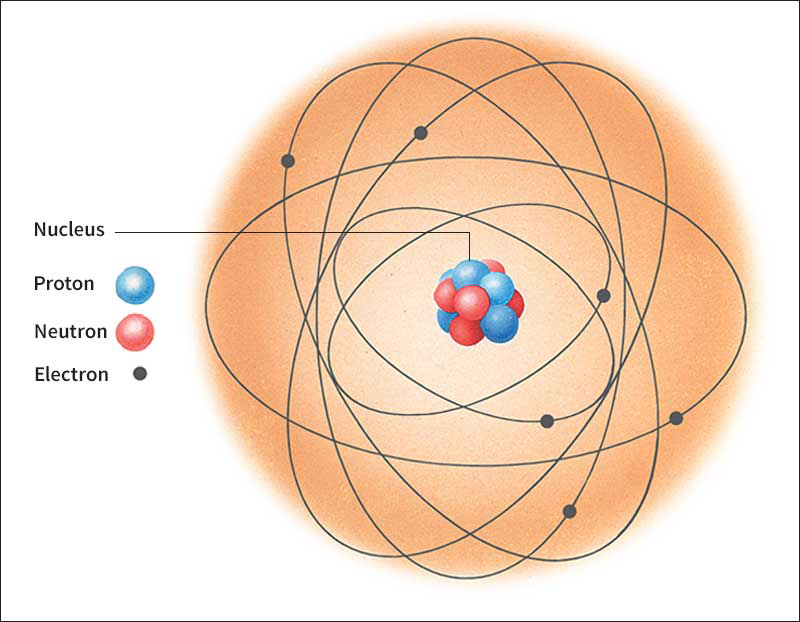
Fundamental ideas of chemistry.
The simplest chemical substances are the chemical elements. They are the building blocks of all other substances. Each chemical element is made up of only one kind of atom. The atoms of one element differ from those of all other elements. Chemists use letters of the alphabet as symbols for the elements. The symbols for the element carbon, for example, is C. Hydrogen has the symbol H. Oxygen and iron are O and Fe. There are 91 elements known to exist on Earth. An additional 20 elements have been produced artificially. See Element, Chemical.
Electrical forces at the atomic level create chemical bonds. These bonds join two or more atoms together, forming molecules. Some molecules consist of atoms of a single element. Oxygen molecules, for example, are made up of two oxygen atoms. See Molecule.
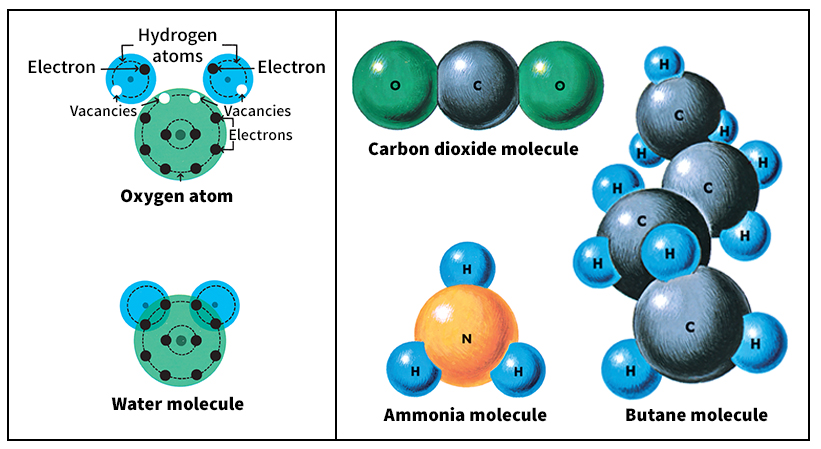
When atoms of two or more different elements bond together, they form a chemical compound. Water is a compound made up of two hydrogen atoms and one oxygen atom. See Compound.
Compounds are formed or broken down by means of chemical reactions. All chemical reactions involve the formation or destruction of chemical bonds. Chemists use chemical equations to express what occurs in chemical reactions. Chemical equations consist of formulas and symbols that show the substances involved in chemical changes.
The broad range of study.
Chemists study substances according to questions they want to answer. Many chemists study special groups of substances. For example, they might study compounds containing carbon-to-carbon bonds. Some chemists specialize in techniques that enable them to analyze any substance. They can then identify the elements and compounds a substance consists of. Other chemists study the forces involved in chemical changes. Much chemical research deals with the atomic and molecular structures of substances. Certain chemists try to predict chemical behavior from theories about the forces at work within the atom. Chemists work to create new substances. They also try to make synthetic forms of rare but useful natural materials. Their field is called synthetic chemistry. A number of chemists apply their knowledge to agriculture, industry, medicine, and other fields.
In some cases, chemistry overlaps other sciences. Such sciences as biology, geology, mathematics, and physics overlap chemistry to such an extent that interdisciplinary sciences have been established. Biochemistry, for example, combines biology and chemistry in studying the chemical processes of living things.
Tools and techniques.
Chemists use a wide variety of tools and techniques. Specialized instruments and computers help chemists make accurate measurements. A device called a mass spectrometer, for example, enables chemists to determine the mass and atomic composition of molecules. Mass is the total quantity of matter that anything contains. Chemists can identify how atoms are arranged in molecules by using instruments that measure the radiation absorbed and given off by the molecules. The measurement technique is called spectroscopy. A technique called chromatography enables chemists to separate complicated mixtures into their parts. They can also detect and measure low concentrations of substances, such as pollutants in air and water.
History of chemistry
Beginnings.
In prehistoric times, people made many useful discoveries by observing the properties of natural substances and the changes those substances go through. About 11/2 million years ago, people began to use fire. Fire was the first chemical reaction that human beings learned to produce and control. The use of fire enabled people to change the properties of substances. They used fire for cooking, hardening pottery, and making metal from ores. Fire also enabled them to create new materials. About 3500 B.C., for example, people learned to make bronze by melting together copper and arsenic—and later copper and tin.
The people of many ancient cultures believed that gods or spirits caused natural events. About 600 B.C., however, some Greek philosophers began to regard nature in a different way. They believed that nature worked according to laws that people could discover by observation and logic.
Several ancient Greek philosophers developed theories about the basic substances that make up the world. Empedocles, who lived during the 400’s B.C., argued that there were four primary elements—air, earth, fire, and water. He believed that the four elements combined in various proportions to form all other substances.
About 400 B.C., a Greek philosopher named Democritus taught that all matter was composed of a single material. The material existed in the form of tiny, indestructible units called atoms. According to his theory, differences among substances were caused only by differences in the size, shape, and position of their atoms.
The Greek philosopher Aristotle, who lived during the 300’s B.C., followed Empedocles. Aristotle claimed that each of the four primary elements proposed by Empedocles could be changed into any of the other elements by adding or removing heat and moisture. He stated that such a change—called transmutation—occurred whenever a substance was involved in a chemical reaction. Transmutation also took place whenever a substance changed from one physical state—solid, liquid, or gas—to another. Aristotle believed that water, for example, changed to air when it was heated.
Alchemy.
During the first 300 years after the birth of Christ, scholars and craftworkers in Egypt developed a chemical practice that came to be called alchemy. They based their work on Aristotle’s theory of the transmutation of elements. Alchemists tried to change lead and other metals into gold. Alchemy began to spread to the Arabian Peninsula in the A.D. 600’s and to much of western Europe in the 1100’s. Until the 1600’s, alchemy was a major source of chemical knowledge.

Despite centuries of experimentation, alchemists failed to produce gold from other materials. They did gain wide knowledge of chemical substances, however. They also invented many tools and techniques still used by chemists. Alchemists used such laboratory equipment as funnels, strainers, and crucibles (pots for melting metals). They also used balance scales for weighing chemicals—that is, for determining the chemicals’ mass. Alchemists discovered new ways of producing chemical changes. They also learned to make and use various acids and alcohols.
Alchemists also searched for a substance that could cure disease and lengthen life. During the 1500’s, some alchemists and physicians began to apply their knowledge of chemistry to the treatment of disease. The medical chemistry of the 1500’s and 1600’s is called iatrochemistry << eye `at` roh KEHM uh stree >> . The prefix comes from iatros, the Greek word for physician. Iatrochemists made the first studies of the chemical effects of medicines on the human body.
Robert Boyle, an Irish scientist of the 1600’s, was one of the first modern chemists. He taught that theories must be supported by careful experiments. Boyle conducted many experiments that showed that air, earth, fire, and water are not true elements. He believed that the best explanation of the properties of matter was provided by an atomistic theory that described substances as composed of tiny particles in motion.
The phlogiston theory
was developed in the early 1700’s. A German chemist and physician named Georg Ernst Stahl wrote that all flammable materials contained a substance called phlogiston << floh JIHS tuhn >> . According to his theory, materials gave off phlogiston as they burned. Air was necessary for combustion because it absorbed the phlogiston that was released. Plants, in turn, removed phlogiston from the air. They therefore became rich in the substance and burned when dry. The phlogiston theory provided an explanation for the results of a variety of experiments. It also offered clues to areas of study in which new discoveries could be made. For those reasons, the theory was widely accepted in the 1700’s. It led to many findings in chemistry.
Chemists of the middle and late 1700’s developed ways to isolate and study gases. They based their work on the phlogiston theory and made many discoveries. In the 1750’s, the Scottish chemist and physician Joseph Black identified carbon dioxide. Carbon doxide was the first gas recognized to have properties different from those of air. In 1766, Henry Cavendish, an English chemist and physicist, discovered important properties of hydrogen. Cavendish identified hydrogen as an element. Because hydrogen is flammable, he believed it was pure phlogiston. Oxygen was discovered independently by the Swedish chemist Carl Scheele in the early 1770’s and the English chemist Joseph Priestley in 1774. Wood burns stronger in oxygen than in air. Thus, Priestley believed oxygen could absorb great quantities of phlogiston. He called oxygen dephlogisticated air (air without phlogiston).
Lavoisier’s contributions.
Antoine Lavoisier, a French chemist, revolutionized chemistry in the late 1700’s. He repeated many of the experiments of earlier chemists but interpreted the results differently. Lavoisier paid particular attention to the mass of the ingredients involved in chemical reactions. He compared that mass with the mass of the products that resulted. He found that the mass of the products of combustion equals that of the original ingredients. His discovery became known as the law of the conservation of mass (or matter).

Lavoisier noted that the mass of the air in which combustion occurred decreases. He found that the loss of mass results from the burning material combining with and removing a substance in the air. That substance was the same as dephlogisticated air. But Lavoisier renamed it oxygen. Lavoisier’s oxygen theory of combustion came to replace the phlogiston theory.
Lavoisier and Pierre Simon Laplace, a French astronomer and mathematician, also carried out experiments involving respiration in animals. They showed that respiration is chemically similar to combustion. Their studies of the chemical processes of living organisms were among the first experiments in biochemistry. Lavoisier also helped work out the present-day system of chemical names. He presented his ideas on combustion, respiration, and the naming of compounds in a book. This book, Elementary Treatise on Chemistry (1789), was the first modern textbook of chemistry.
Dalton’s atomic theory.
In 1803, an English chemist named John Dalton developed an atomic theory based on the idea that each chemical element has its own kind of atoms. He believed that all the atoms of a particular element had the same mass and chemical properties. The theory could explain and predict the results of various experiments. Other scientists gradually accepted Dalton’s ideas.

According to Dalton’s theory, a fixed number of atoms of one substance always combined with a fixed number of atoms of another substance in forming a compound. Dalton realized that substances must combine in the same proportions by mass as the mass proportions of their atoms. Chemists had already observed that pure substances do combine in fixed proportions. They called that finding the law of definite (or constant) proportions. Dalton’s theory explained the law.
Dalton was the first to calculate the relative masses of the atoms of several elements. By 1814, Jons J. Berzelius, a Swedish chemist, had obtained accurate atomic masses for a number of elements. He also began the system of using letters of the alphabet as symbols for elements.
Formation of the periodic table.
The Russian chemist Dmitri Mendeleev created what scholars widely consider to be the first modern periodic table. The table was published in 1869. It is based on the observation that when elements are arranged according to their relative atomic masses, elements with similar properties appear at regular intervals, or periods, in the table. Mendeleev arranged the elements based primarily on their atomic mass. But he also switched the order of some elements on the basis of their chemical properties. In addition, he left gaps in his table where no known elements seemed to fit. Mendeleev correctly predicted that elements with certain properties would be discovered to fill the gaps. The German chemist Julius Lothar Meyer independently developed a similar periodic table. However, his table was not published until 1870. The modern periodic table serves as a guide to the chemistry of all known elements. See Periodic table.

Development of organic chemistry.
From the time of the alchemists, researchers had investigated various substances found in plants and animals. Such organic substances, however, proved difficult to analyze. As a result, nearly all early chemical knowledge was obtained by studying simpler inorganic substances.
Most chemists of the early 1800’s believed that organic compounds could be produced only with the aid of a vital force, a life force present in plants and animals. That belief is called vitalism. In 1828, a German chemist named Friedrich Wöhler mixed two inorganic substances and heated them. By this process, he obtained urea—an organic compound found in urine. Wöhler thus made the first synthetic organic substance from inorganic materials. He proved that no vital force is needed to produce organic compounds.

During the 1800’s, chemists isolated many organic substances. They discovered that most organic compounds consist mainly of carbon combined with hydrogen, nitrogen, and oxygen in various proportions. In 1831, German chemist Justus von Liebig developed a simple, accurate technique for analyzing organic compounds. About the same time, chemists found that, in certain cases, two organic compounds with different properties are composed of the same elements in the same proportions. Berzelius called such compounds isomers. Isomers have the same kinds and numbers of atoms. But they differ in how the atoms are joined.
In the mid-1800’s, chemists developed the valence theory to explain how atoms combine and form molecules. Valence refers to the normal number of bonds one atom can form with other atoms. In 1858, a German chemist named Friedrich Kekulé von Stradonitz proposed that carbon atoms can bond to four other atoms. He also suggested that certain carbon atoms can link to form chains. As a result of his ideas, chemists recognized organic compounds as molecules based on a framework of carbon-to-carbon bonds.
By 1900, the study of organic substances had become a major branch of chemistry. Chemists have since learned how to produce numerous complex organic molecules.
In the mid-1900’s, Melvin Calvin, an American chemist, solved many long-standing mysteries of photosynthesis. Photosynthesis is the chemical process by which plants make food. Since the mid-1900’s, biochemists have discovered how such complex organic substances as deoxyribonucleic acid (DNA) and ribonucleic acid (RNA) affect heredity (see DNA; Heredity; RNA).
Development of physical chemistry.
During the 1800’s, many chemists and physicists investigated the properties of substances and the energy changes that accompany chemical reactions. They based their work on ideas about the structure and behavior of atoms and molecules. Such study is called physical chemistry.
One of the first scientists to explore the area of physical chemistry was Amedeo Avogadro, an Italian physicist. In 1811, Avogadro suggested that equal volumes of all gases with the same temperature and pressure contain equal numbers of particles. His idea is now known as Avogadro’s law. It helped chemists calculate relative atomic masses. Later in the 1800’s, physical chemists developed the kinetic molecular theory. This theory describes solids, liquids, and gases as clusters of particles that are in constant motion. It explains how the movement of these clusters at high speeds determines the pressure, temperature, and other properties of gases.
During the mid-1800’s, physicists formulated the principles involved in the conversion of heat into mechanical energy and vice versa. They thereby laid the foundations for chemical thermodynamics. Chemical thermodynamics is the study of the changes in heat that accompany many reactions.
During the 1870’s, an American scientist named Josiah Willard Gibbs developed the phase rule. The rule explains the physical relationships among the solid, liquid, and gaseous phases (states) of matter. Jacobus van’t Hoff, a Dutch chemist, relied on the phase rule in his studies of how crystals form in solutions. Van’t Hoff’s work led to the development of stereochemistry, the study of the arrangement of atoms in molecules.
In the late 1800’s, physical chemists Svante A. Arrhenius of Sweden and Wilhelm Ostwald of Germany proposed that electric current is carried through solutions by charged atoms or molecules called ions. Ostwald wrote one of the first textbooks in electrochemistry, the study of chemical changes associated with electrical forces.
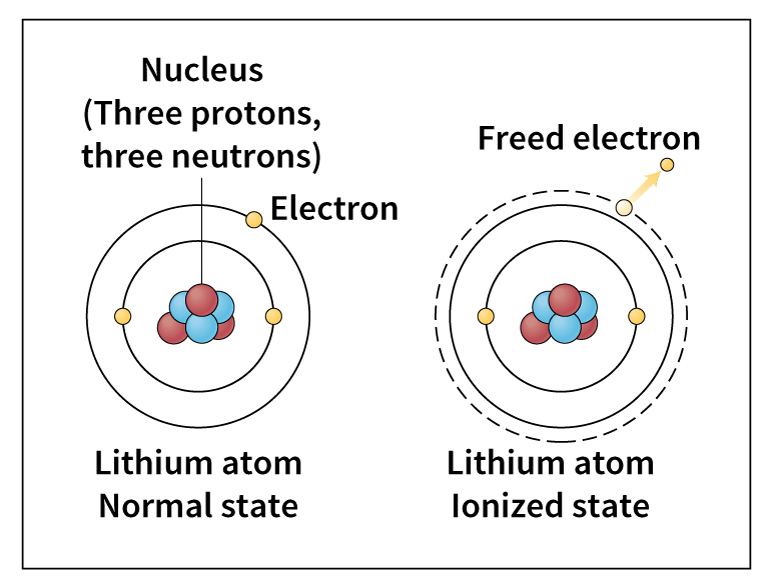
Since the early 1900’s, chemists and physicists have devoted much study to the structure of atoms and molecules. In 1913, a Danish physicist named Niels Bohr suggested a model of the atom. In Bohr’s model, electrons are arranged in successively larger orbits around a small nucleus of protons and neutrons. He believed many properties of an element depend on the number of electrons in the outer orbit of the atoms of that element.
Bohr’s model of the atom also helped explain how atoms interact with light and other forms of radiation. Bohr assumed that the absorption and emission (giving off) of light by an atom involve a change in the energy state of an electron. As a result, the electron jumps from one orbit to another. Chemists have gained much information about the structure of molecules by measuring their absorption and emission of radiation.
In 1916, Gilbert N. Lewis, an American chemist, proposed that the bond between atoms in a molecule consists of a pair of electrons that both atoms share. His idea led to the electron pair theory. This theory explains the bonding characteristics of elements in terms of the arrangement of their electrons. See Bond [chemical].
Growth of industrial chemistry.
The use of chemical knowledge by manufacturers started with the origins of chemistry itself. During the 1700’s, however, manufacturers of such products as acids, alkalis, and soap began to use the knowledge of chemists on a broad scale. With a knowledge of chemistry, they could improve their products and production methods. During the 1800’s, factories turned out huge quantities of such chemicals as sulfuric acid, sodium carbonate, and bleaching powder. In 1856, the English chemist Sir William H. Perkin produced mauve, also called aniline purple—the first synthetic dye. Its popularity soon led to the synthesis of other dyes for the textile industry.
By 1900, Germany had the most advanced chemical industry in the world. In 1910, a German chemist named Fritz Haber patented a process to produce ammonia from hydrogen and nitrogen. His work led to the large-scale manufacture of chemical agricultural fertilizers. During World War I (1914-1918) and World War II (1939-1945), the chemical industry expanded greatly in several countries. The industry grew to meet the demand for such war materials as explosives, medications, and synthetic rubber.
After World War II, the chemical industry continued to produce a great variety of goods for consumers. The development of new materials resulted in the widespread use of plastics and such synthetic fibers as nylon and polyesters. In addition, further discoveries led to the availability of many new drugs, food preservatives, fertilizers, and pesticides.
Current research.
Biochemistry is an active area of scientific research today. New instruments have enabled biochemists to study the action of chemicals within an organism without harming the organism. Biochemists are studying substances suspected of causing cancer or genetic damage. Such studies may help determine what molecular features are responsible for the harmful effects. Other chemists are investigating how chemical pollutants affect the environment and how they break down into other substances.
Synthetic chemistry is another area of active research. Chemists synthesize many thousands of new compounds each year. They have discovered chemical agents that can be used in reactions to add special groups of atoms to specific parts of other molecules. Researchers design new molecules and use such agents in a series of reactions to build the new compounds. Their techniques have led to the creation of many drugs.
The study of the surface properties of chemical compounds—called surface chemistry—is another promising field of present-day research. Chemists have learned that surface characteristics are responsible for the ability of certain substances—called catalysts—to speed up the rate of chemical reactions. Chemists today are also working to develop a chemical cell that would use the energy of sunlight to break up water molecules into oxygen and hydrogen. The hydrogen thus produced could be used as fuel. Such cells may one day provide a valuable new source of energy.
The chemical industry
The chemical industry plays a vital role in the production of many manufactured goods. The industry provides a tremendous variety of materials to other manufacturers. It also produces many chemical products that benefit people directly. Major products of the industry include such household products as detergents, drugs, paper products, plastics, and synthetic fibers. Other chemical products include dyes, fertilizers, food preservatives and flavorings, glass, and metal alloys.

Most major chemical products are basic chemicals used in the manufacture of other products. Sulfuric acid is the chief basic chemical in the United States and many other countries. It is used to produce fertilizers and numerous other chemicals. Other basic chemicals include chlorine, nitrogen, and oxygen. Such alkalis as lime and sodium hydroxide are basic chemicals. So are the chemicals used in plastics.
The production of chemicals has become increasingly concentrated in multinational companies. Such companies have plants and offices in a number of countries. To help keep costs low, the companies tend to locate their factories in countries where raw materials are readily available. Many basic chemicals are produced in developing countries by factories of multinational firms. But chemicals requiring advanced production methods are made mainly in developed countries.
Most chemical companies have research and development programs. Chemists in those programs work to develop new substances, new uses for known chemicals, and improvements in chemical production techniques.
The success of the chemical industry has been accompanied by environmental and safety problems. For example, the use of huge amounts of pesticides has resulted in soil and water pollution. In addition, the production of some chemicals results in harmful waste products that must be disposed of safely. Many chemical dumps for the storage of such wastes have leaked, threatening the health of people in nearby areas. During the late 1900’s, a number of accidents occurred at chemical plants in several countries. These accidents resulted in the release of harmful substances.
Chemical companies have had to spend much money in efforts to solve environmental and safety problems. For example, they are working to develop insecticides that will quickly break down into harmless substances in the environment. They are also seeking safer methods of disposing of chemical wastes and of cleaning up chemical dumps. In addition, the companies are increasing safety precautions at chemical plants to guard against accidents.
Careers in chemistry
Chemistry offers a variety of challenging career opportunities in education, industry, and government. High school and college courses helpful to students preparing for a career in chemistry include mathematics and physics as well as classes in chemistry. Many chemical instruments make use of computer technology, and so classes in computer science are also useful. Writing courses help chemists develop their ability to communicate scientific information to others.
A bachelor’s or master’s degree in chemistry is sufficient for some careers, including teaching chemistry in junior high and high schools. Some chemists with advanced degrees teach at universities or conduct research. A doctor’s degree is important for students who wish to pursue basic research—that is, the study of fundamental laws and processes of chemistry.
Many university graduates with specialized knowledge in chemistry find employment in industry. They work as plant superintendents, chemical engineers, quality control personnel, and salespeople. In addition, a large number of chemists are hired by government agencies involved in such areas as trade, environmental protection, and public health.
