Chlorine << KLAWR een >> is a poisonous, yellowish-green gas with a strong, unpleasant odor. Chlorine causes irritation to the nose, throat, and lungs. However, when combined with the metal sodium, chlorine forms sodium chloride, or table salt.
The Swedish chemist Carl Wilhelm Scheele first made chlorine in 1774 by treating muriatic acid (hydrochloric acid) with manganese dioxide. In 1810, the English chemist Sir Humphry Davy determined that chlorine was a chemical element. He named it from a Greek word meaning greenish-yellow.
Sources of chlorine.
In nature, chlorine exists only in compounds. It is found mainly in chloride minerals, of which the best known is sodium chloride. Chlorides occur in seawater, salt lakes, and deposits of rock salt.
Uses of chlorine.
Chlorine kills bacteria in water and so it is widely used to purify drinking water and the water in swimming pools. In sunlight, chlorine can react explosively with hydrogen to form hydrogen chloride. This compound dissolves in water to become hydrochloric acid. People use hydrochloric acid in dyeing and in cleaning metal. When chlorine is dissolved in sodium hydroxide, it becomes a mixture of sodium chloride and sodium hypochlorite. This mixture has often been used as a bleach and disinfectant.
Manufacturers also use chlorine compounds to produce paper, plastics, insecticides, cleaning fluids, and antifreeze. In addition, chlorine is used in the manufacture of medicines, paints, and petroleum products.
Manufacturers produce chlorine gas chiefly by passing an electric current through solutions of sodium chloride in water (see Electrolysis ). Sodium hydroxide (also called caustic soda, or lye) is formed at the same time. This process is the basis of one of the largest chemical industries, the chlor-alkali or chlorine-caustic industry. Chlorine can be put under pressure and made into a liquid.
Chemical properties.
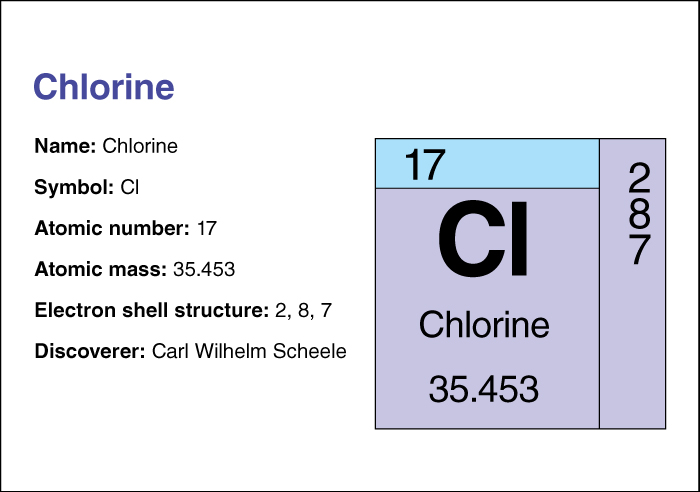
Chlorine is a member of the halogen (salt-forming) group of nonmetallic elements. Pure chlorine is extremely active chemically. Like the other halogens, it tends to combine with other elements by accepting electrons from them. Chlorine acts as a powerful oxidizing agent by causing substances to give up electrons. Chlorine has the chemical symbol Cl. It has an atomic number (number of protons in its nucleus) of 17. Its relative atomic mass is 35.4527. An element’s relative atomic mass equals its mass (amount of matter) divided by 1/12 of the mass of carbon 12, the most abundant form of carbon. At 20 °C, chlorine gas has a density of 0.00295 grams per cubic centimeter at sea level. Chlorine may be condensed to a liquid that boils at –34.05 °C and freezes at –100.98 °C. For information on the position of chlorine on the periodic table, see the article Periodic table .
