Distillation, << `dihs` tuh LAY shuhn, >> is a process that separates a substance or a mixture of substances from a solution through vaporization. Many industrial processes depend on distillation. Distillation usually involves boiling a liquid and condensing the vapor that forms.
Water that is treated by this process forms distilled water. This water is purer than the original water because salts and other impurities do not evaporate with the water.
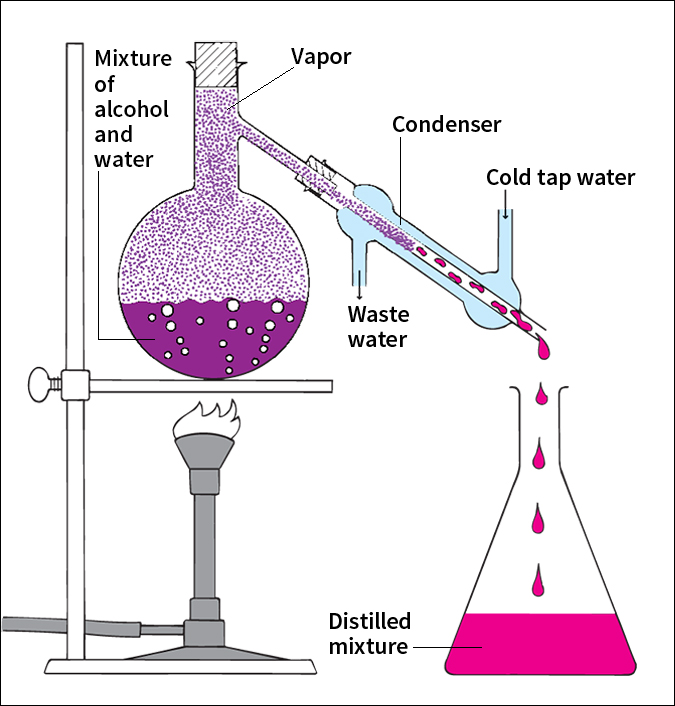
Distillation can be carried out in an apparatus called a still. A still consists of a boiler, a condenser, and a receiver. The mixture to be vaporized is heated in the boiler. Whichever substance in the mixture boils at the lowest temperature will be the first to turn into vapor. The vapor enters the condenser, where it cools and condenses back into liquid. The distilled liquid, called the distillate, then collects in the receiver. The other components of the solution remain in the boiler.
Two general methods are used to distill liquids, simple distillation and rectification. In simple distillation, all the distillate is removed from the still after collecting in the receiver. In rectification, part of the distillate flows back into the still. This portion comes into contact with the vapor being condensed and enriches it.
Simple distillation.
Two common techniques used in this method of distillation are fractional distillation and flash distillation.
Fractional distillation,
also called differential distillation, separates a mixture of liquids that boil at different temperatures. For example, ethanol (grain alcohol) boils at 172 °F (78 °C), and water boils at 212 °F (100 °C). When a mixture of these liquids is heated, the alcohol vaporizes faster than the water. But the water also vaporizes at the boiling point of alcohol. As a result, the distillate from a mixture of alcohol and water contains some water. The first distillate collected has a larger proportion of alcohol than the portions that condense later. Therefore, the first distillate is removed before much water distillate has condensed. In the same way, the remaining distillate is collected in fractions (portions), which can then be redistilled to make a purer product. Fractional distillation is used in making distilled liquors and in refining petroleum. See Alcoholic beverage ; Distilling .
Flash distillation
involves passing a liquid from a vessel maintained at a high pressure to one kept at a lower pressure. No heating is required to produce vapor by this method. The lower pressure causes part of the liquid to flash (turn quickly) into vapor, which is then condensed into distillate. In fractional distillation, the distillate can be processed only in batches. But in flash distillation, a continuous flow of liquid can be distilled. Flash distillation is widely used to turn ocean water into fresh water. See Water (Distillation) .
Rectification
separates many different substances from a solution by using large towers called fractionating columns. As the mixture is heated, its vapors rise through these columns. Substances that boil at the lowest temperatures form the first fractions. Their vapors rise highest and are carried off by pipes near the tops of the fractionating columns. Pipes at different heights along the column carry off fractions that contain different substances. The reflux (return) of some distillate to the columns produces the most efficient conditions for this method of distillation. Like flash distillation, rectification can be carried out with a continuous feed of liquid. Rectification plays an important role in chemical processing, including petroleum refining. See Petroleum (Refining petroleum) .
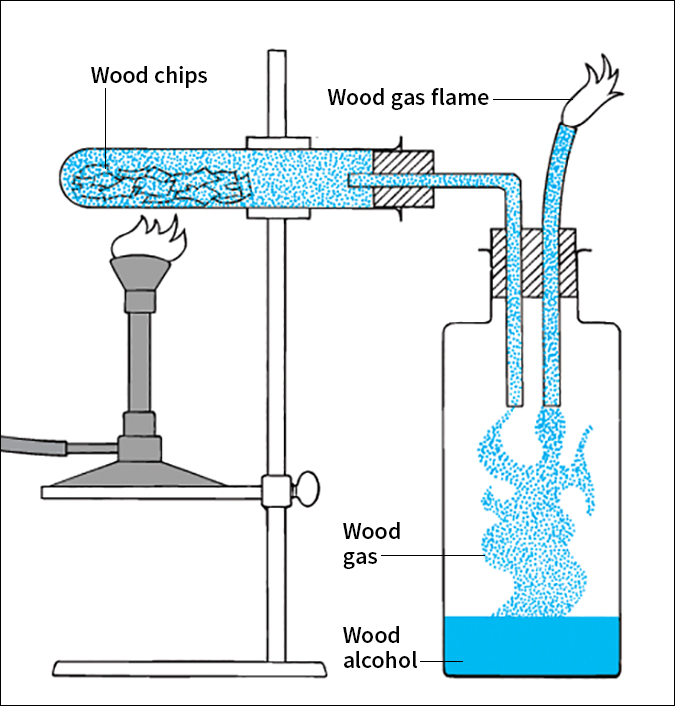
Destructive distillation.
No new substances are formed during simple distillation or rectification. Each of these processes simply separates substances that have been mixed together. But when some solids are heated in a closed vessel, they decompose (separate chemically) or react to produce new substances. For example, wood heated in an airtight tube decomposes into wood gas, which in turn condenses and forms wood alcohol. This process, which involves chemical changes, is called destructive distillation. Manufacturers use destructive distillation to produce coal tar from coal.