Electrolysis << ih `lehk` TROL uh sihs >> is a process in which an electric current passes through a liquid, causing chemical reactions to occur. If the liquid is water, electrolysis breaks water molecules into hydrogen and oxygen. If the liquid contains metals, electrolysis removes the metals from the liquid. Electrolysis has many practical uses, especially in industry.
How electrolysis works.
Electrolysis takes place in a device called an electrolytic cell. An electrolytic cell consists of two solid electrical conductors, called electrodes, placed in a liquid. Most electrodes are metal or carbon rods. The liquid may be water or another substance that conducts electric current. The liquid may contain dissolved substances called electrolytes that increase its ability to conduct electric current.
Wires connect the electrodes to the terminals of a battery or another source of direct current (current that flows in one direction). The electrode connected to the incoming current is called the cathode. It transfers electrons, which are negatively charged, from the battery to the electrolytic cell. The other electrode is called the anode. It transfers electrons out from the electrolytic cell and back to the battery. The movement of electrons produces an electric current.
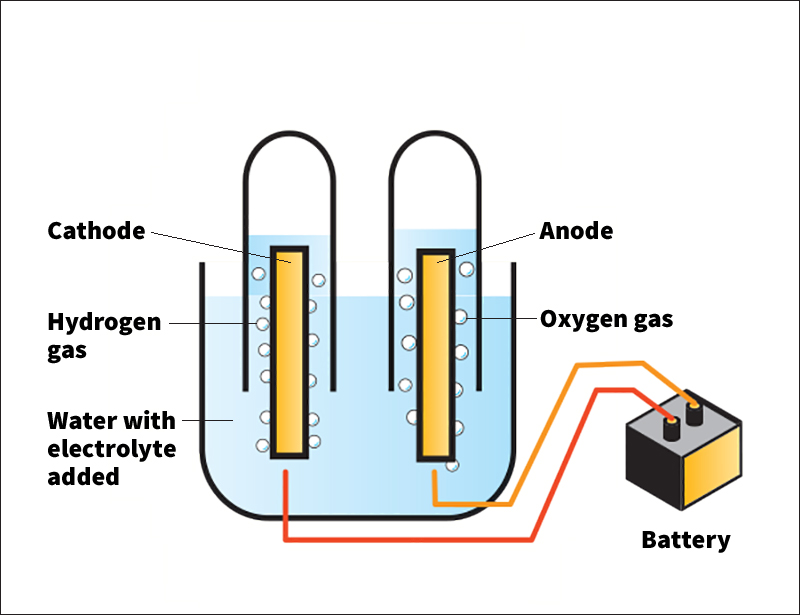
The liquid in the electrolytic cell contains ions. Ions are atoms or molecules that have lost or gained an electron, becoming positively or negatively charged. Positive ions are attracted to the cathode of the electrolytic cell, and negative ions are attracted to the anode. The movement of ions carries electric current between the electrodes.
At the cathode, electrons are transferred to atoms or molecules in the liquid. This process, called reduction, causes chemical reactions to take place. For example, if a water molecule near the cathode gains an electron, one of its hydrogen atoms splits off and sticks to the cathode. It soon joins another hydrogen atom, forming a molecule of hydrogen gas. The hydrogen gas bubbles out of the water. If the liquid contains ions of a metal, such as gold or silver, reduction removes the metal from the solution, causing a thin layer of the metal to form on the cathode.
At the anode, electrons are transferred from atoms or molecules in the liquid and join the current flowing back into the battery. This transfer, called oxidation, also causes chemical reactions. For example, when water gives up electrons at the anode, oxygen atoms split from the water molecules and join to make oxygen gas. The gas bubbles out of the water.
Uses of electrolysis.
Electrolysis is used to produce many chemical substances in pure form. For example, electrolysis of ordinary salt (sodium chloride) produces pure sodium metal. Aluminum and magnesium metals can be produced in a similar way. Because metal ions carry a positive electric charge, the metals collect at the negatively charged electrode. Chlorine and other chemicals are also produced by electrolysis. Because chlorine ions carry a negative charge, chlorine collects at the positively charged electrode, where it bubbles out as a gas.
Electrolysis can also purify an impure substance. For example, impure copper can serve as the anode in an electrolytic cell, and pure copper can serve as the cathode. Chemical reactions at the anode transfer positively charged copper ions to the liquid. These ions are attracted to the cathode. The copper ions stick to the copper cathode, increasing the amount of pure copper.
A kind of electrolysis called electroplating coats a metal object’s surface with a thin layer of another metal. In electroplating, the metal that is to form the coating is dissolved in the liquid. The object to be coated is used as the cathode. This process can make an object appear more attractive or provide a surface that resists damage.
A similar process, called anodizing, coats a layer of protective oxides (oxygen compounds) on a metal used as the anode in an electrolytic cell. Manufacturers often make cookware and outdoor furniture from aluminum that has been anodized to protect it from corrosion.
Electrolysis of water produces hydrogen and oxygen gases, which can be captured as the gases bubble up at the two electrodes. This process can produce hydrogen for use in fuel cells, devices that convert chemical energy to electrical energy. Some scientists are experimenting with photoelectrolysis, in which sunlight provides the energy to produce hydrogen by electrolysis. Fuel cells powered by hydrogen produced in this manner could provide a clean, renewable energy source.
Principles of electrolysis.
The English chemist Michael Faraday was the first scientist to state mathematical laws describing electrolysis. Faraday’s laws state that the amount of a substance produced in electrolysis depends on the amount of electric charge that passes through the electrolytic cell. The laws also state that the amount of substance produced depends on the weight of an atom or molecule of the substance and the amount of electric charge ions of the substance carry.
The relationship between the substance’s properties and the amount of the substance produced is very precise. This allows scientists to use electrolysis to identify and measure quantities of unknown substances. This technique is called electroanalysis.
