Darmstadtium << dahrm SHTAHT ee uhm or dahrm STAT ee uhm >> is an artificially produced, radioactive chemical element. Darmstadtium has an atomic number (number of protons in its nucleus) of 110. Its name comes from Darmstadt, Germany, the city where it was first produced.
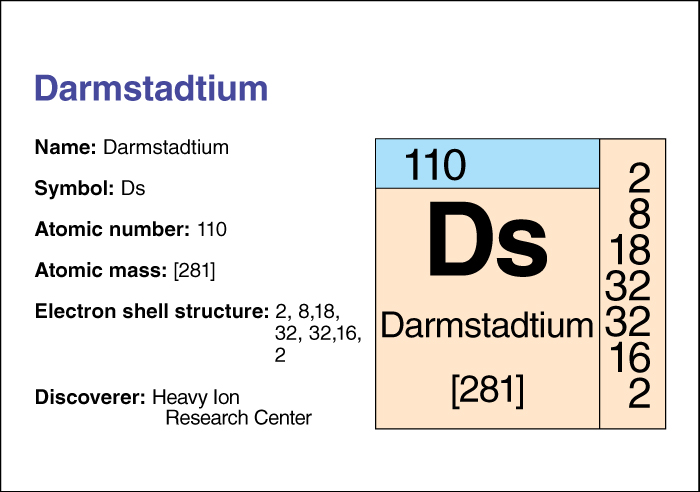
The chemical symbol of darmstadtium is Ds. Chemists place darmstadtium in the transactinide group of transuranium elements. For information on the position of darmstadtium on the periodic table, see the article Periodic table.
Scientists have reported six isotopes of darmstadtium. Different isotopes of an element have the same number of protons but different numbers of neutrons. The atomic mass numbers (total numbers of protons and neutrons) of those isotopes range from 267 to 281.
The atom of darmstadtium with the longest lifetime was an atom of isotope 281—that is, it had an atomic mass number of 281. The atom decayed (broke apart in a radioactive process) in 1.6 minutes.
An international group of scientists working at the Heavy Ion Research Center in Darmstadt first produced darmstadtium in 1994. They used a machine called a particle accelerator to bombard lead (atomic number, 82) with nickel (atomic number, 28). The first atom of darmstadtium produced was an atom of isotope 269. That atom decayed 0.00039 seconds after it was first observed.
