Gay-Lussac, << `gay` luh SAK, >> Joseph Louis (1778-1850), was a French chemist and physicist. In 1802, he published the results of his experiments dealing with the effects of temperature on gases. His findings agreed with the earlier unpublished work of French chemist Jacques A. C. Charles and are usually known as Charles’s law (see Gas (Gas laws) ). He studied the chemistry of gases and, in 1809, summarized data collected by others in the law of combining volumes. This law states that gases form compounds with each other in simple, definite proportions. These proportions can then be expressed in formulas. The formula for water shows it is formed of two parts of hydrogen and one of oxygen.
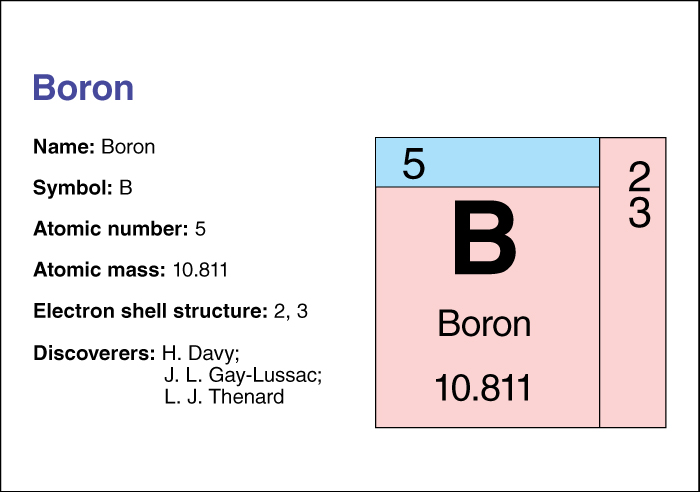
Gay-Lussac investigated the upper atmosphere. In 1804, he rose about 23,000 feet (7,000 meters) above sea level in a hydrogen-filled balloon. He wanted to study the composition, temperature, and moisture of air and the strength of the earth’s magnetic pull at high altitudes. Gay-Lussac improved the processes for making sulfuric acid and oxalic acid for industry. He also suggested improved ways of estimating the amount of chlorine in bleaching powder. Gay-Lussac and Louis Jacques Thenard prepared potassium and sodium from potash and soda. The two men also independently isolated boron in the same year (1808) that Sir Humphry Davy of England did (see Boron ). Gay-Lussac discovered the gas cyanogen in 1815. He was born on Dec. 6, 1778, in Leonard-le-Noblat. He died on May 9, 1850.
