Antimony, << AN tuh `moh` nee, >> a chemical element, is a bluish-white, brittle metalloid. It is used to harden and strengthen lead. Antimony-lead alloys are used in some electric cables and batteries. Compounds containing antimony are used in producing materials used in refrigerators, air conditioners, aerosol sprays, paints, and flame-proofing agents.
Antimony is most commonly found combined with sulfur in the mineral stibnite. Pure antimony can exist as metallic antimony—the more common form—and as a black powder, known as black or gray antimony.
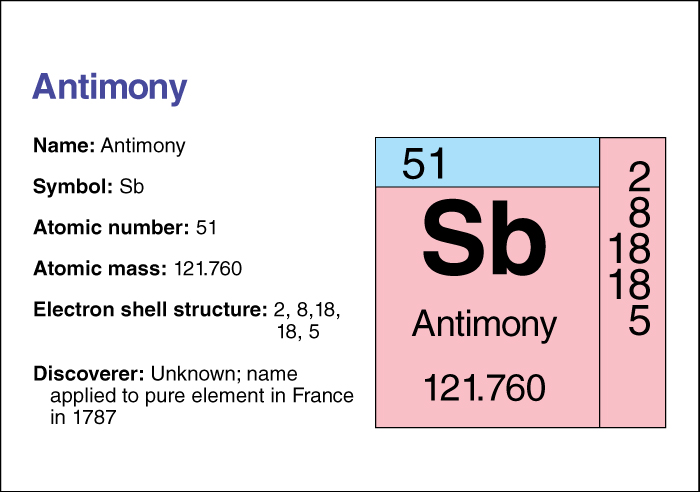
Antimony has the chemical symbol Sb, from stibium, the old name for the element. Its atomic number (number of protons in its nucleus) is 51. Its relative atomic mass is 121.760. An element’s relative atomic mass equals its mass (amount of matter) divided by 1/12 of the mass of carbon 12, the most abundant form of carbon. The density of antimony is 6.691 grams per cubic centimeter at 20 °C. Antimony melts at 630.74 °C and boils at 1586.85 °C. It conducts (carries) electric current better as a liquid than as a solid. For information on the position of antimony on the periodic table, see the article Periodic table .
Antimony has been used for thousands of years. The ancient Egyptians used substances containing antimony as cosmetics and medicines. The Bible and ancient writings from China, India, and Mexico also refer to medical uses of antimony preparations.
See also Element, Chemical (tables) ; Lead .
