Hydrolysis, << hy DROL uh sihs, >> is a chemical reaction involving water as one of the reacting substances. Its name comes from two Greek words meaning water and loosening. In industry, hydrolysis is important in making soap, sugar, alcohols, hydroxides, and silicones.
Hydrolysis produces either of two chemical changes: (1) the acidity of the reacting system may change, or (2) molecules of both water and another substance may split and recombine to form new substances.
An example of hydrolysis that increases the acidity of a system is the reaction of antimony chloride (SbCl3) with water. This hydrolysis produces antimony oxychloride and hydrochloric acid. In the chemical equation, the presence of the acid is indicated by the hydronium ion (H3O+). This equation is written:
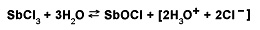
An example of hydrolysis that decreases the acidity of a system is the reaction of sodium carbonate (Na2CO3) with water. This hydrolysis forms a mixture of sodium ions (Na+), bicarbonate ions (HCO3 –), and hydroxide ions (OH–). The chemical equation is written:
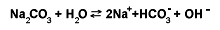
An example of hydrolysis in which molecules of both water and another substance split and recombine differently is the reaction of sucrose (cane sugar, C12H22O11) with water in the presence of acid. This hydrolysis produces two simpler sugars, glucose and fructose. These simpler sugars have the same chemical formula (C6H12O6), but they differ in molecular structure. The chemical equation for the hydrolysis of sucrose is:
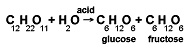
The hydrolysis of sucrose is an important part of digestion. Sucrose cannot be used by the body, but glucose and fructose can.
