Argon, << AHR gon, >> is a chemical element that forms 0.94 percent of Earth’s atmosphere. Most ordinary light bulbs are filled with argon and a little nitrogen. Argon is also used as a shielding gas in arc welding to protect the metal from oxygen in the air. The English physicist Lord Rayleigh and the Scottish chemist Sir William Ramsay discovered it in 1894. See Ramsay, Sir William ; Rayleigh, Lord .
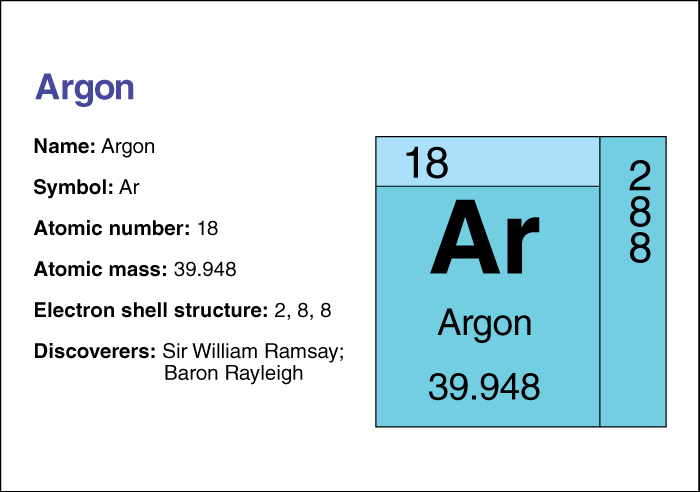
Argon is a colorless, odorless, tasteless gas. It does not react readily with other chemicals. Only one compound of the element is known to exist. The compound, called argon fluorohydride, is only stable below –256 °C. This chemical element is classed as a noble gas (see Noble gas ). Its symbol is Ar. Its atomic number (number of protons in its nucleus) is 18. It has a relative atomic mass of 39.948. An element’s relative atomic mass equals its mass (amount of matter) divided by 1/12 of the mass of carbon 12, the most abundant form of carbon. The freezing point of argon is –189.2 °C, and its boiling point is –185.7 °C. Its density is 0.00178 gram per cubic centimeter at 0 °C and one atmosphere of pressure (see Atmosphere ). For information on the position of argon on the periodic table, see the article Periodic table .
Argon is continuously released into the atmosphere through the decay (breaking down) of radioactive potassium in Earth’s crust. When the potassium decays, it changes into argon. Commercially, argon is obtained as a by-product in the manufacture of liquid air (see Liquid air ).
