Indium, a chemical element, is a rare, soft, silver-white metal. Engineers coat the bearings of high-speed engines with indium, which causes oil to spread over the bearings evenly. Engineers also add indium to gallium nitride in the making of lasers and devices called light-emitting diodes (LED’s). Lasers made in this way produce a blue light used to read high-definition DVD’s.
Indium combines with oxygen to form indium oxide, a light yellow solid. Chemists add tin oxide to indium oxide to make indium tin oxide, abbreviated ITO. Thin films of ITO conduct electric current well and appear transparent. These qualities make ITO useful in such devices as computer touch screens and liquid crystal displays (LCD’s). In the 2000’s, demand for ITO in flat-screen televisions caused the price of indium to surge higher than that of silver. Engineers also use ITO as a reflective coating in energy-saving windows and light bulbs.
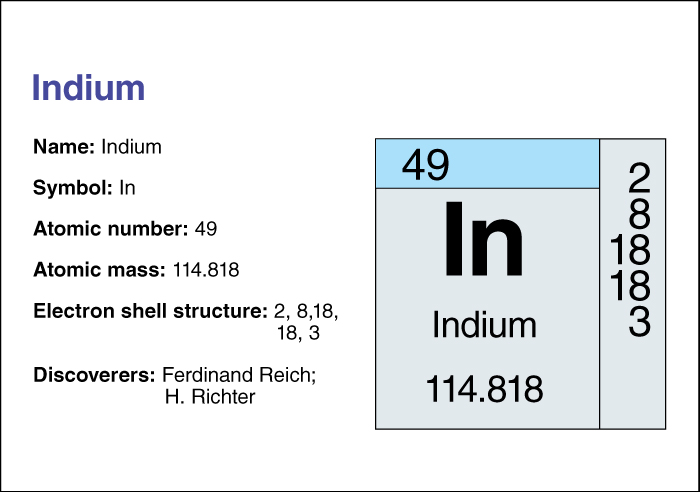
Indium’s atomic number (number of protons) is 49. Its relative atomic mass is 114.818. An element’s relative atomic mass equals its mass (amount of matter) divided by 1/12 of the mass of carbon 12, the most abundant form of carbon. Indium melts at 156.61 °C and boils at 2080 °C. Chemists classify indium as an other metal . For information on the position of indium on the periodic table, see the article Periodic table .
Indium does not occur by itself. Most of it is found in, and extracted from, certain zinc ores. The German scientists Ferdinand Reich and Hieronymus Theodor Richter discovered the element in 1863.
