Isotope, << EYE suh tohp, >> is one of two or more atoms of the same chemical element that differ in the amount of matter they contain. All of an element’s isotopes have the same number of protons (positively charged particles) but a different number of neutrons (electrically neutral particles). Some elements, such as fluorine, gold, and phosphorus, have only one naturally occurring isotope.
Most elements have several naturally occurring isotopes. For example, hydrogen has three isotopes. Its lightest and most abundant isotope, called protium, has only a single proton in its nucleus. The second isotope, called deuterium or heavy hydrogen, has one proton and one neutron in its nucleus. The heaviest hydrogen isotope, tritium, has a nucleus that consist of one proton and two neutrons. See Atom (The atomic number) (The atomic mass number) (Relative atomic mass) .
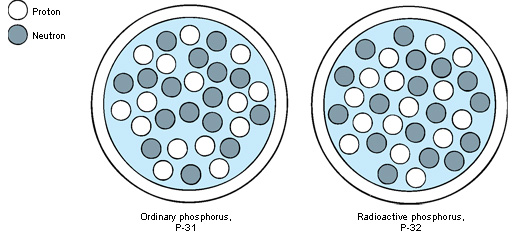
The number of protons in the nucleus of the atoms of an element determines the element’s atomic number. Thus, the atomic number of hydrogen is 1. No two elements have the same atomic number. Uranium, the heaviest element found in sizeable amounts in nature, has 92 protons. Its atomic number is 92. The total number of protons plus neutrons equals an isotope’s atomic mass number. All the isotopes of an element have a different atomic mass number. For example, protium has a mass number of 1, deuterium has a mass number of 2, and tritium has a mass number of 3.
Scientists use symbols to identify isotopes. For uranium (chemical symbol, U), the isotope of atomic mass number 235 may be written 235/92U. Since all atoms of an element have the same atomic number, it may be omitted: 235U, which may also be written U-235. In addition, an isotope may be indicated by the name of the element followed by the isotope’s atomic mass number. For example, “uranium 235” is another way of indicating the isotope U-235.
Some elements have many naturally occurring isotopes. Tin, for example, has 10. The lightest tin isotope is Sn-112 and the heaviest is Sn-124. The most abundant tin isotope is Sn-120, which makes up about a third of the element. The least abundant is Sn-115. Only 0.34 percent of the tin atoms are this isotope. Except for a few special cases, the relative proportions of the different isotopes in a sample of an element are always the same, no matter where the sample comes from.
Radioactive isotopes.
More than 270 stable isotopes occur in nature. About 50 other natural isotopes, including those of uranium and radium, are radioactive. These isotopes, which give off particles or radiations, are called radioisotopes.
All the elements heavier than bismuth (atomic number 83) are radioactive. They decay (break down) into lighter-weight isotopes of other elements and belong to three radioactive decay series, which begin with U-238, U-235, and the thorium isotope Th-232. These three heavy atoms decay into various isotopes until they eventually become stable isotopes of lead. The rate at which radioactive isotopes decay is measured by the half-life, or the time required for half the atoms in a sample to decay. Every isotope has a specific half-life. Some isotopes in the radioactive series decay slowly. For example, the radium isotope Ra-226 has a half-life of 1,600 years. Others decay much faster. Some have half-lives of a small fraction of a second. Isotopes that have short half-lives can occur naturally because they are continually being formed by the decay of the parent (heaviest) isotope of the series.
A few scattered radioactive isotopes that do not belong to the series exist among elements lighter than bismuth. These elements include potassium 40, rubidium 87, samarium 146, lutetium 176, and rhenium 187.
Separating isotopes.
In the early 1940’s, during World War II, scientists developed processes for separating large quantities of various isotopes. The separation of uranium isotopes and hydrogen isotopes has proved especially useful. For example, U-235 is separated from the more abundant U-238 for use in atomic bombs and various nuclear reactors. Similarly, deuterium, H-2, has to be separated from the abundant light hydrogen isotope, H-1, for use in hydrogen fusion research and for other purposes (see Fusion ).
The methods of separating deuterium from light hydrogen depend on the fact that deuterium is twice as heavy as light hydrogen. The rate of a chemical reaction depends on the mass of the element involved. The relative difference between the masses of the two hydrogen isotopes is large. Therefore, a reaction involving deuterium proceeds at a different rate from that of a reaction involving light hydrogen. By making use of this principle, scientists separate deuterium from light hydrogen on a large scale. They produce large quantities of deuterium each year. The relative difference in mass between boron 10 and boron 11 is also large enough for this method of separation.
The relative difference in mass between the various uranium isotopes is so small that scientists must use other methods to separate them. The most successful method is called gaseous diffusion. This method depends on the fact that in a gas, a heavier molecule moves somewhat more slowly than a lighter one. As a result, in a gaseous compound that contains uranium, a molecule containing the lighter isotope passes more easily through tiny holes in a porous sheet. If this process is repeated several thousand times in a row, a useful amount of the lighter isotope becomes separated. Huge laboratories in the United States and other countries separate large quantities of uranium isotopes by this method.
Pure isotopes of most elements are available in small amounts for experimental purposes. These isotopes are produced by still another method, which can be adapted for use with many elements. An electrical discharge ionizes a vapor of the element or of a compound containing the element. In ionization, one of the electrons that orbit around the atom’s nucleus is knocked off. The atom then has a positive charge.
An electric field accelerates the charged atoms, called ions, to a definite energy. This process produces a beam of ions, in which all the ions have the same energy. When a magnetic field bends the ion beam, the ions with different masses separate into circles of different radii. Each circle consists of a different isotope of the element. The process takes place in a container from which the air has been removed. Scientists used this method during World War II to separate uranium isotopes. But the gaseous diffusion method proved less expensive. A somewhat similar process, called mass spectrometry, is used to measure the relative abundance of naturally occurring isotopes and to make precise determinations of nuclear masses (see Mass spectrometry ). A number of other methods have also been used to separate isotopes.
Artificial radioisotopes.
Scientists have artificially produced many radioisotopes that are not found naturally on Earth. These artificial isotopes can be produced either in cyclotrons and other particle accelerating devices or in nuclear reactors. For example, scientists may bombard an isotope of sodium, Na-23, with high-energy deuterons in a cyclotron. A deuteron, derived from a deuterium atom, is a particle made up of a proton and a neutron. When a deuteron collides with a Na-23 atom, a nuclear reaction occurs. The deuteron’s neutron becomes part of the nucleus of the atom and a proton is ejected, producing Na-24. See Particle accelerator . Radioisotopes are also made by exposing elements to the large number of neutrons in a nuclear reactor, a process called neutron capture. For example, in a reactor, Na-23 atoms capture neutrons and become Na-24. Radioisotopes can result from the fission (splitting) of atoms. The fission (splitting) of uranium leads to the formation of more than 450 radioactive isotopes and over 100 stable isotopes.
Although common isotopes typically have from roughly 1 to 11/2 times as many neutrons as protons, some rare artificial isotopes have many more neutrons than protons. For example, scientists have created an isotope of aluminum, Al-42, that has 13 protons and 29 neutrons. However, a nucleus with a given number of protons can hold only so many neutrons. Researchers are working to determine the maximum number of neutrons that each element can have.
Scientists have produced about 1,700 radioisotopes. Artificial isotopes of all the elements have been produced. For many elements, 15 or more artificial isotopes are known.
All elements not found naturally on Earth have been artificially produced. These include technetium and promethium, which are present in some stars, and the transuranium elements, elements 93 through 110 (see Transuranium element ). These elements are radioactive, and they have such short half-lives that they are not to be found on Earth. An exception is plutonium. Scientists have found small amounts of the plutonium isotope Pu-244 on Earth.
Uses of radioisotopes.
Radioisotopes have many important uses in science and industry. Because they are radioactive, they can be easily detected, even in very small amounts. They are identical chemically with other isotopes of the same element, so they can take the place of the common isotopes in chemical reactions. Thus, they can be used to study the details of chemical or biological reactions. For example, biochemists use radioactive carbon to trace the path of carbon atoms in the photosynthesis process in green plants. They detect the particles and rays emitted by the radioactive atoms with such devices as Geiger counters, gamma-ray spectrometers, and proportional counters.
Radioisotopes are widely used in nuclear medicine, which employs radioactive materials to study, diagnose, and treat certain diseases. Radioisotopes also are used in various kinds of environmental studies, particularly those concerned with nuclear radiation.
In industry, radioisotopes are often used to measure the thickness of materials. The radiation emitted by radioisotopes is partially absorbed in passing through materials. Radiation detectors are used to measure the intensity of the radiation that has passed through the materials. Variations in the intensity of the radiation indicate differences in the thickness of a material being inspected.
