Lanthanide, also called a lanthanoid, is any of the 15 elements that follow the element barium in the periodic table. In order of atomic number, the lanthanides are lanthanum (atomic number 57), cerium (58), praseodymium (59), neodymium (60), promethium (61), samarium (62), europium (63), gadolinium (64), terbium (65), dysprosium (66), holmium (67), erbium (68), thulium (69), ytterbium (70) and lutetium (71). Scientists sometimes do not group lanthanum among the lanthanides.
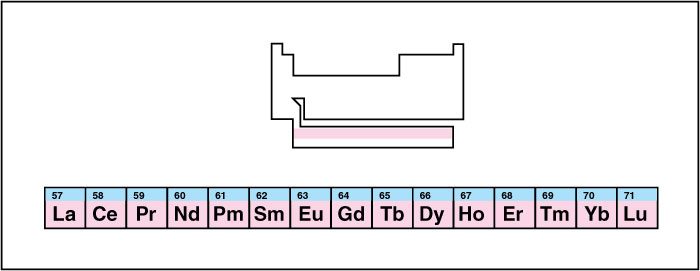
In most periodic tables, the lanthanides and a group called the actinides are shown separate from the rest of the elements in two long rows. If the rows were shown in place, the table would be too wide for easy display.
Lanthanides have an increasing number of electrons in a region of the atom called the 4f subshell. Lanthanum has 0 electron in this subshell, and lutetium has 14. In most cases, each lanthanide has one more electron in the 4f subshell than the previous lanthanide. However, several lanthanides do not follow this pattern, leading to unique electron arrangements.
Lanthanides have similar chemical properties. For example, in forming compounds, lanthanides tend to lose three electrons, gaining a 3+ charge.
People once called the lanthanide elements the rare-earth elements, in part because they were thought to exist at low concentrations in Earth’s crust. But they are now known to be more abundant. All lanthanide elements are present in nature except promethium.
The electrons in a lanthanide atom can exist in a complex pattern, enabling lanthanides to give off or absorb light in particular ways. For this reason they appear in lasers and lenses. Other uses for them include magnets, pigments, lighters, and X-ray screens.
