Astatine << AS tuh teen or AS tuh tihn >> is the heaviest member found in nature of the halogen family of chemical elements. It is radioactive and has the chemical symbol At and an atomic number (number of protons) of 85. For information on the position of astatine on the periodic table, see the article Periodic table.
Chemists think astatine may be the rarest naturally occurring element on Earth, with less than one ounce (28 grams) existing at any one time. Astatine has more than 30 known isotopes, forms with the same number of protons but different numbers of neutrons. The most stable isotope has an atomic mass number (total number of protons and neutrons) of 210. That isotope has a half-life of 8.1 hours—that is, due to radioactive decay, only half the atoms in a sample of isotope 210 would still be atoms of that isotope after 8.1 hours. The chemical properties of astatine resemble those of iodine.
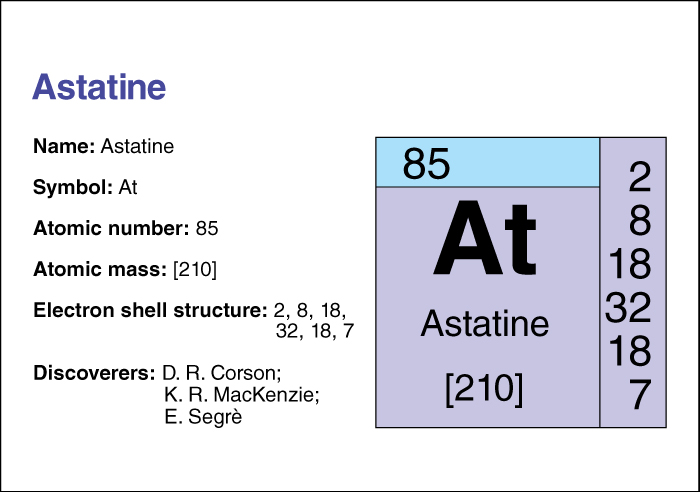

Astatine was first prepared in 1940 by Dale R. Corson, K. R. MacKenzie, and Emilio Segrè at Berkeley, California. It was produced in a cyclotron by bombarding bismuth with high-energy alpha particles. They named it for the Greek word meaning unstable, in reference to its short half-life. Although small amounts of the element exist in uranium ores, almost all astatine is created artificially.
See also Halogen.
