Acid is any of a group of chemical compounds with certain similar properties. Solutions of acids have a sour taste and produce a prickling or burning sensation if they come into contact with the skin. They dissolve many metals and turn blue litmus paper red. Chemical compounds called bases or alkalis neutralize acids.
Many acids occur naturally and some are essential for life. For example, hydrochloric acid (HCl) is produced in the stomach of most people and helps digestion. Acids are also used widely in industry, and they are a part of a large number of foods and beverages. But many acids are poisonous, and strong acids can cause severe burns.
Chemists use several definitions to describe the behavior of acids. When water is the solvent, an acid is often defined as a compound that dissolves to produce hydrogen ions (H+) in solution. A hydrogen atom consists of one proton, which has a positive electric charge, and one electron, which has a negative charge. A hydrogen ion is the proton that remains when a hydrogen atom loses its electron. In solution, the proton is closely associated with solvent molecules, forming hydronium ions (H3O+).
An acid may also be defined as a substance that serves as a proton donor—that is, it readily gives up a proton to another substance. However, acids are most broadly defined as compounds that are electron pair acceptors. This definition describes all acids, including those that have no hydrogen that they can release and that cannot serve as proton donors. The acid accepts a pair of electrons from another atom or molecule. In such cases, the acid and the electron pair donor form a new molecule in which they share the electrons.
The strength of an acid
depends on the degree to which the acid dissociates (breaks up) in solution to form hydrogen ions. For example, in water solution, every molecule of hydrogen chloride (HCl) releases a hydrogen ion to form hydrochloric acid. Hydrochloric acid is therefore considered a strong acid. Acetic acid (CH3COOH), which is found in vinegar, forms only a few hydrogen ions in solution. It is a weak acid.
Inorganic acids,
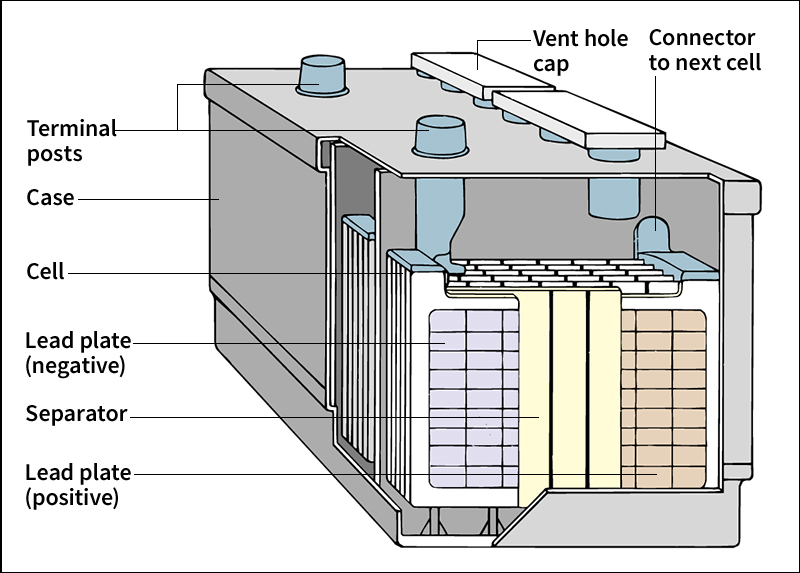
in general, do not contain carbon atoms. Many inorganic acids are strong acids. They are used in the production of other chemicals, explosives, fertilizers, metals, paints, plastics, and synthetic fibers, and in the refining of petroleum. Sulfuric acid (H2SO4), a strong inorganic acid, is commonly used as the fluid in automobile batteries. Other important inorganic acids are hydrochloric acid and nitric acid (HNO3).
Organic acids
contain carbon atoms. They are used in beverages, cosmetics, detergents, foods, drugs, plastics, and soaps. Common organic acids include citric acid (C6H8O7), which is found in citrus fruits; ascorbic acid (C6H8O6), or vitamin C; and acetylsalicylic acid (C9H8O4), or aspirin. Amino acids, which contain nitrogen, are also organic acids. Amino acids are the building blocks of proteins, and some of them are necessary for human life.
