Actinium, << ak TIHN ee uhm, >> a chemical element , is an extremely rare, silvery-white, radioactive metal that glows in the dark. A radioactive element decays (breaks down) by sending out particles from its nucleus. In this process, it changes into another element. Natural actinium is itself a product of radioactivity. It is created by the decay of an isotope of uranium known as uranium 235. An element’s isotopes have the same number of protons but different numbers of neutrons in their nucleus.
Actinium also can be artificially prepared from radium treated with neutrons in a nuclear reactor. It is a difficult element to study because it can be produced only in small quantities and because it decays into products that give off radiation.
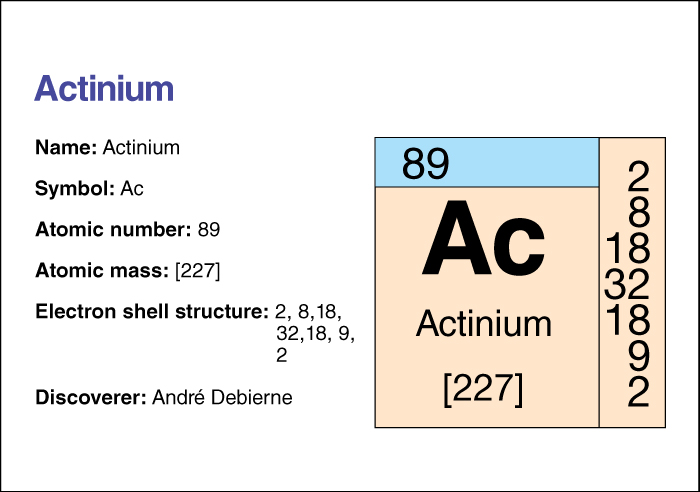
Actinium has the chemical symbol Ac. Its atomic number (number of protons) is 89. Its most stable isotope has an atomic mass number (total number of protons and neutrons) of 227. That isotope has a half-life of 21.77 years—that is, due to radioactive decay, only half the atoms in a sample of actinium 227 would still be atoms of that isotope after 21.77 years (see Isotope ). Actinium melts at 817 °C and boils at 2470 °C. Most simple actinium compounds, such as the oxides, hydrides, and halides, contain the positively charged actinium ion, Ac3+.
Andre Debierne, a French scientist, discovered actinium in 1899. For information on the position of actinium on the periodic table, see the article Periodic table .
