Air is the mixture of gases that surrounds Earth. Air covers the land and sea and extends far above Earth’s surface. We cannot see, smell, or taste air. But when the wind blows, it is the air you feel against your face. Wind is simply moving air. You can also see the effect of wind in drifting clouds, pounding waves, and trembling leaves. Moving air can turn windmills and blow large sailboats across the ocean. The word air is often used interchangeably with the word atmosphere. However, atmosphere refers to the air and all the elements in the air, such as clouds, dust, and smoke.
Everyone needs air to stay alive. People have lived more than a month without food and more than a week without water. But a person can live only a few minutes without air.
Air does much more than make it possible for us to breathe. Air shields Earth from most of the harmful radiation from the sun. At the same time, gases in the air absorb heat emitted (given off) by the ground that has been warmed by the sun. In this way, air helps keep Earth warm enough to support life. It shields our planet from meteors, most of which burn up in the atmosphere before striking the ground.
We also need air to hear. Sound must travel through the air or some other substance. Most of the sounds we hear travel through the air. Thus, the world would be silent if there were no air. Air has weight. This weight enables balloons filled with a light gas or heated air to rise high above Earth. They rise because they are lighter than the air around them. Air moving over the wings of airplanes, birds, and insects enables them to fly.
The quality of the air we breathe depends largely on the amount of pollutants (impurities) that people add to the atmosphere. Air pollution is a serious problem in most of the world’s big cities. Motor vehicles and industrial processes produce most of the wastes that cause air pollution. But many other pollutants that affect air quality come from natural sources, such as volcanic ash, smoke from forest fires, pollen, and dust. Polluted air can harm the health of living things. It can also damage building materials and even affect the weather.
What is air?
Air consists of a mixture of gases that extends from Earth’s surface to outer space. Earth’s gravitational pull prevents the air from escaping out into space. The gases of the air move freely among one another. As sunlight passes through Earth’s atmosphere, it strikes molecules of the gases. These molecules scatter the sunlight, which is a mixture of all colors, in every direction. The sky appears blue because the gas molecules scatter more blue light than any other color. See Sky .
Many small particles of dust are suspended among the gases of the air. The air also carries tiny water droplets and ice crystals in the form of clouds. Scientists do not consider dust, water droplets, and ice crystals to be part of the air. Scientists do consider these suspended substances to be a part of Earth’s atmosphere.
Gases of the air.
The principal gases of the air are nitrogen and oxygen. Other gases include argon, water vapor, carbon dioxide, methane, neon, helium, krypton, hydrogen, xenon, and ozone. The water vapor in the air is water in the form of an invisible gas. Nitrogen makes up about 78 percent of dry air—that is, air from which all water vapor has been removed. Oxygen accounts for about 21 percent of dry air. The remaining 1 percent consists chiefly of argon, with only extremely small amounts of the other gases, often called trace gases. Except for water vapor, the gases in the atmosphere tend to be thoroughly mixed as high as an altitude of about 60 miles (100 kilometers).
Some gases of the atmosphere are particularly important. When we breathe, we take in oxygen from the air and give off carbon dioxide. Green plants take in carbon dioxide and give off oxygen in a food-making process called photosynthesis (see Photosynthesis ). Most fuels must have oxygen to burn. Certain bacteria turn nitrogen that has passed into the soil from the atmosphere into chemicals that fertilize plants.
Water vapor and carbon dioxide are two of the primary gases in the air that help retain Earth’s heat. They absorb infrared radiation (heat) emitted by the ground and oceans. In this way, the gases prevent some of the surface heat created by sunlight from escaping back into space. Ozone, a form of oxygen, absorbs many of the sun’s harmful ultraviolet rays, an invisible form of light that has been linked to skin cancer.
Moisture in the air
takes the form of water vapor and tiny particles of liquid water and ice. Water vapor enters the atmosphere when water evaporates from oceans, lakes, rivers, and soil. Water is also given off from the leaves of plants in a process called transpiration. The sum total of evaporation and transpiration is called evapotranspiration.
The amount of vapor in the air depends on location. For example, air over oceans and in the tropics usually contains more vapor than air over the interior of continents or near the North and South poles. Furthermore, air near Earth’s surface typically contains more vapor than air several miles or kilometers above the ground. The amount of water vapor in the air also depends on weather patterns and winds. For instance, air normally contains more vapor if it is blowing from an ocean.
Warm air can hold much more water vapor than cold air. Scientists express the ability of air to hold water vapor in terms of relative humidity—the air’s vapor content divided by its vapor capacity. Vapor capacity is often defined as the maximum amount of vapor the air could hold (see Humidity ). According to this definition, air that is holding as much water vapor as possible has a relative humidity of 100 percent. Air just above water surfaces, such as the ocean, often has a relative humidity near 100 percent. Air over dry surfaces, such as deserts, usually has a low relative humidity.
However, air in clouds can actually have a relative humidity greater than 100 percent. If air is cooled enough, its vapor capacity decreases. The excess vapor can then change to tiny water droplets in a process called condensation or ice crystals in a process called deposition. The temperature at which water vapor begins to condense into tiny liquid droplets is called the dew point.
Air cools as it rises. Clouds form when masses of moist air rise and cool to below the dew point. The vapor in clouds condenses on tiny particles of matter called cloud condensation nuclei. These particles include sea salt, dust, soot, or such chemical compounds as ammonium sulfate or magnesium sulfate. Most ice crystals form in cold clouds as water vapor deposits onto tiny particles called ice nuclei.
Clouds consist of air filled with millions of water droplets, ice crystals, or both. Rain or snow develops after the growing droplets or crystals become heavy enough to fall out of the clouds. Fog is a cloud near Earth’s surface.
Particles in the air.
Air always contains many tiny solid particles called aerosols. Most aerosols measure only about 1/250,000 inch (0.1 micrometer) in diameter. They are therefore invisible, except when crowded together in extremely large numbers.
Many aerosols enter the air from active volcanoes, automobile exhaust, forest and brush fires, and factory smoke. The wind carries particles of dust and sand up from the ground into the atmosphere. Other aerosols include pollen from plants, salt from the oceans, ashes of meteoroids, and tiny living things called microbes. In the presence of high humidity and sunlight, sulfur dioxide, a by-product of burning fossil fuels, turns into aerosols of sulfuric acid. In summer, a build-up of these aerosols may turn the sky a hazy shade of white.
Aerosols are always being added to the air. But they do not remain in the atmosphere forever. Rain and snow wash out many aerosols, so air is often fresher after it rains or snows. Other aerosols slowly fall to Earth.
Near Earth’s surface, the number of aerosols in the air varies greatly from place to place. The air over the oceans contains about 30 million aerosols per cubic foot (1 billion per cubic meter). However, the polluted air over a large city may contain about 3 billion aerosols per cubic foot (100 billion per cubic meter). Fewer aerosols float in the higher regions of the atmosphere. Thus, the air in mountainous regions is usually purer.
How air behaves
Weight and pressure.
We do not usually notice the weight of air because air is much lighter than solids or liquids. At sea level, each cubic foot of air weighs only about 1 1/5 ounces, a measurement equivalent to about 1.2 kilogram per cubic meter. But the weight of all the air around the world is more than 5,700 trillion tons (5,200 trillion metric tons). The gravitational pull of Earth maintains the atmosphere and prevents the air from escaping into outer space. The weight of the air pressing from the top of the atmosphere upon the layers of air below produces air pressure, also called atmospheric pressure. The air pressure at sea level averages 14.7 pounds per square inch (101.3 kilopascals). The air pressing down on your shoulders weighs about 1 ton (0.9 metric ton). You do not feel this weight because you are supported by equal air pressure on all sides.
An instrument called a barometer is used to measure air pressure. Air pressure is usually lower on stormy, wet days than on clear, dry days. Thus, a falling reading on a barometer often indicates that a storm is coming. See Barometer .
The upper atmosphere has less pressure than the air near Earth, simply because there is less air pressing down from above. When you ride up a tall building in a fast elevator, you can feel the air pressure changing. As you rise higher, the pressure of the air inside the elevator decreases, but the air pressure inside your ears remains the same. This difference in pressure causes your eardrums to bulge outward slightly until some air finally forces its way out of your ears, causing your ears to “pop.”
We use the pressing force of air in various ways. When we sip a soft drink through a straw, for example, we do not actually pull the liquid up through the straw. Instead, by sucking on the straw, we remove some of the air from inside it. As a result, the air pressure inside the straw becomes less than the pressure of the air on the liquid outside the straw. The greater pressure of the air outside then pushes the liquid up through the straw and into our mouths. Suction pumps and vacuum cleaners also work by means of air pressure (see Pump ; Vacuum cleaner ).
Air movement.
Air moves across the surface of Earth in the form of wind blowing from regions of high pressure to low pressure. These local areas of high and low pressure are primarily a result of the sun’s uneven heating of Earth. Air above warm areas of Earth expands and becomes lighter. It then rises, creating an area of low pressure near the surface. Wind is produced when cooler, heavier air flows toward the low-pressure area, replacing the rising air. Wind often develops along an ocean shore during the day because land heats up more quickly than water does. The air over the shore is thus warmer than the air over the water. As the warm air over the shore rises, the cooler air from the sea moves inland and replaces it, producing a daytime sea breeze. At night, the air over the shore becomes cooler than the air over the water. Thus, the wind direction reverses, and a breeze blows out to sea as a land breeze.
The warm air above the equator is usually rising. Cooler air from north and south of the equator blows in steadily, replacing the rising air. This movement of air creates two vast belts of winds called the trade winds. The trade winds do not blow straight toward the equator because of Earth’s rotation. The trade winds north of the equator are twisted to the right of their original direction, or toward the southwest. The trade winds south of the equator are shifted to their left, or toward the northwest. The alteration of wind flow due to Earth’s rotation is called the Coriolis force or Coriolis effect. The Coriolis force also effects the flow pattern of two other great belts of winds that circle Earth, the prevailing westerlies and polar easterlies. See Trade wind ; Coriolis effect ; Wind .
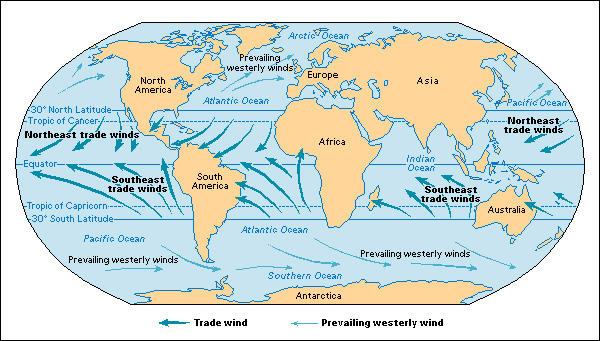
Bands of fast-moving winds occur about 6 to 9 miles (10 to 15 kilometers) above Earth. These bands are known as jet streams. Winds in the core of a jet stream may exceed 200 miles (320 kilometers) per hour. Systems of swirling winds, called cyclones and anticyclones, also form in the air. The winds of cyclones swirl inward toward a center of low pressure. Anticyclones whirl outward around a center of high pressure. See Jet stream ; Weather (Synoptic-scale systems) .
Air resistance.
Air resists the motion of objects traveling through it. This resistance occurs because moving objects rub against the atoms and molecules of the gases that make up the air. A feather in the air floats slowly to the ground because of air resistance acting on its surface. Air resistance also slows a parachute jumper’s fall.
During the early days of aviation, airplanes flew slowly partly because such parts as the wing braces and landing wheels rubbed against the air. Aviation engineers found that they could reduce air resistance and thus increase the speed of a plane by streamlining its shape. They removed outside wing supports and installed landing wheels that could be pulled up into the plane. They found that even smoothing down rivet heads helped reduce air resistance. See Aerodynamics .
The faster objects move through the air, the more resistance they meet. For example, the faster you ride a bicycle, the stronger the air resistance against you will be. As you increase your speed, you can feel the air pushing harder against you. This air resistance generates heat, but you will actually feel cooler because the faster-moving air removes heat from your body more effectively. However, for objects traveling at great speeds, the heat generated by air resistance exceeds the cooling effect of the wind. Meteoroids speeding through the atmosphere encounter great air resistance and thus become hot enough to glow, producing streaks of light known as meteors. Most disintegrate before hitting the surface. Rockets traveling through Earth’s atmosphere must be made of materials that can withstand the intense heat created by air resistance.
Air compression.
Air can be pumped into cylinders or tanks until the air pressure is several hundred times greater than normal atmospheric pressure. Such air is called compressed air. When air is being compressed, the atoms and molecules of the air speed up. As their speed increases, the air gets warmer.
People use compressed air to inflate tires and air mattresses. Scuba divers breathe from tanks of compressed air strapped to their backs. Submarines carry cylinders of compressed air. A submarine dives as compartments called ballast tanks are flooded with water. It rises to the surface as the water is forced out of the ballast tanks by compressed air. Compressed air is also used to operate air brakes, certain insecticide and paint sprayers, and air hammers and other pneumatic tools (see Pneumatic tool ).
Structure of the atmosphere
Scientists divide Earth’s atmosphere into four layers according to differences in temperature. These layers, from the lowest to highest altitude, are (1) the troposphere, (2) the stratosphere, (3) the mesosphere, and (4) the thermosphere. The atmosphere becomes thinner with increasing height above Earth. This thinning occurs because the number of air molecules in a given amount of space decreases. The outer atmosphere gradually fades into space, where it meets the solar wind—that is, a continuous stream of charged particles from the sun. See Solar wind .
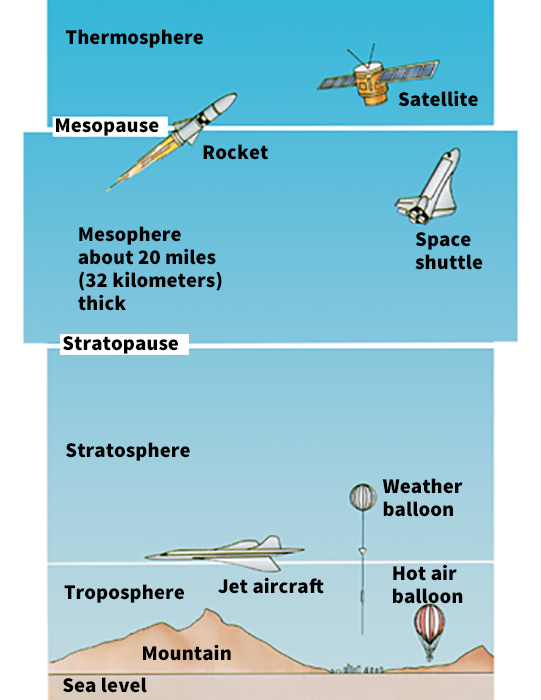
The troposphere
is the layer of the atmosphere closest to Earth—the layer in which we live. The troposphere contains more than 75 percent of Earth’s air. Nearly all Earth’s weather conditions—including most clouds, rain, and snow—occur in this layer. Scientists forecast the weather by studying the behavior of the troposphere. Most of the aerosols and water vapor in the air are in the troposphere. Jet streams blow in the upper part of the troposphere.
The temperature of the troposphere decreases about 3.5 °F for every 1,000 feet (6.5 °C for every 1,000 meters) of increase in altitude. The temperature stops decreasing at the tropopause, the upper boundary of the troposphere. The tropopause lies about 6 miles (10 kilometers) over the North and South poles and about 12 miles (19 kilometers) over the equator. At the tropopause, the air has become too thin to support life.
The troposphere is usually warmest near Earth’s surface because the majority of sunlight that passes through the air heats the ground and seas. The ground and seas, in turn, warm the air directly above them.
Sometimes, especially at night and during the winter, the air near Earth’s surface becomes cooler than the air above it. This cooling near the ground causes the temperature in a thin layer of the troposphere to increase with altitude. This situation is called a temperature inversion or thermal inversion. The worst outbreaks of air pollution occur during temperature inversions. The warmer air overlying cold air traps pollutants and prevents them from rising and scattering. An inversion typically lasts until sunlight heats the air below or wind breaks up the overlying layer of warm air.
The coldest part of the troposphere is at the tropopause. Temperatures at the tropopause range from about –60 to –112 °F (–50 to –80 °C). The air is so cold that clouds can only exist as ice. The coldest part of the tropopause is over the equator. There, the temperature drops as low as –112 °F (–80 °C). The tropopause can be as much as 54 °F (30 °C) warmer over the poles than over the equator.
The stratosphere
extends from the tropopause to about 30 miles (48 kilometers) above Earth’s surface. Little moisture enters the stratosphere, so clouds are rare. Often airline pilots on large jets prefer to fly in the stratosphere to stay above the weather disturbances in the troposphere. Temperatures are fairly steady in the lower stratosphere, typically hovering between –60 and –70 °F (–50 and –55 °C). Temperatures then increase with height in the rest of the stratosphere, reaching about 28 °F (–2 °C) at the top of the stratosphere, called the stratopause. The stratosphere contains most of the atmosphere’s ozone. Ozone heats the air by absorbing the sun’s ultraviolet rays. This ozone helps protect life on Earth from the harmful ultraviolet radiation of the sun.
The mesosphere
extends from the stratopause to roughly 50 miles (80 kilometers) above Earth. The temperature of the mesosphere decreases with altitude. The lowest temperatures in Earth’s atmosphere occur at the top of the mesosphere, called the mesopause. At the mesopause over the poles, the air temperature can drop below –200 °F (–130 °C) during the summer. Trails of hot gases left by meteors can be seen in the mesosphere. Extremely strong winds blow in this layer. These winds blow from west to east during the winter and from east to west during the summer.
The thermosphere
begins at the mesopause and extends into space. It is the thickest layer of the atmosphere by height. The air in the thermosphere is extremely thin. More than 99.99 percent of the atmosphere lies below it. The chemical composition of the thermosphere differs from that of the lower layers. In the lower regions of the thermosphere, many of the oxygen molecules in the air are broken into oxygen atoms. The outer layer of the thermosphere consists chiefly of hydrogen and helium.
The thermosphere is completely exposed to the sun’s ultraviolet radiation, which heats the thin air there to extremely high temperatures. Ordinarily, the temperature climbs rapidly from the mesopause to about 1100 °F (600 °C) at 120 miles (200 kilometers) above Earth and then levels off. But during periods of peak solar activity, more radiation and particles strike the thermosphere. The increased radiation heats the thermosphere to a temperature as high as 4500 °F (2500 °C).
Much of the thermosphere, along with the upper part of the mesosphere, includes a region called the ionosphere. When radiation from the sun and from other sources in outer space strikes the air in the thermosphere, it ionizes (charges electrically) some of the atoms and molecules of the air. These charged atoms and molecules are called ions. The ionosphere plays an important part in long-distance radio communication because it reflects radio waves back to Earth that would otherwise travel into space. See Ionosphere .
Natural light displays called auroras also occur primarily in the ionosphere. The display in the Northern Hemisphere is called the aurora borealis or northern lights. The display in the Southern Hemisphere is called the aurora australis or southern lights.
The outer portion of the thermosphere, called the exosphere, has so little air that satellites and spacecraft orbiting Earth there encounter almost no resistance. The atoms and molecules of the air in the exosphere move extremely fast. Some travel so fast that they overcome the force of Earth’s gravity and escape into space. Earth is thus slowly losing its atmosphere. However, the process will take billions of years before all the air around Earth disappears.

Origin of the atmosphere
Most scientists believe that Earth formed about 4 1/2 billion years ago and probably did not then have an atmosphere. Slowly, gases that escaped from the developing Earth began to accumulate around it. For example, numerous volcanoes on the young Earth released such gases as ammonia, carbon dioxide, carbon monoxide, hydrogen, methane, nitrogen, sulfur dioxide, and water vapor. These volcanic gases made up a large part of Earth’s earliest atmosphere.
Much of the water vapor from the volcanoes condensed, forming rivers, lakes, and oceans. Some of the other gases in the early atmosphere dissolved in the oceans or combined with rocks on Earth’s surface. But most of the nitrogen stayed in the air. Additional gases, such as argon and xenon, were added by the decay of radioactive elements in Earth.
Before about 2.4 billion years ago, Earth’s atmosphere held almost no oxygen. After 2.4 billion years ago, oxygen began to accumulate. Scientists think that single-celled organisms called cyanobacteria were the source of this initial oxygen. Like plants, cyanobacteria produce oxygen as a by-product of photosynthesis. The level of atmospheric oxygen varied over time. However, it remained well below current levels until about 750 million to 550 million years ago. Then oxygen began increasing to current levels, eventually even exceeding it at times. Scientists think that the increase in oxygen enabled the development of multicellular life. See Earth (History of Earth) .
Changes in the atmosphere
Human activity has caused small but important changes in the composition of the air. The amounts of many gases in the air, such as carbon dioxide, are increasing at significant rates. Carbon dioxide enters the atmosphere whenever coal, oil, or other fuels containing carbon are burned. Since the mid-1700s, the amount of carbon dioxide in the air has increased by about 40 percent, primarily because of the use of such fuels. Levels of methane and nitrous oxide have more than doubled.
Human activity has also added chlorofluorocarbons (CFC’s) to the atmosphere. CFC’s are synthetic substances used as refrigerants in air conditioners and refrigerators and as propellants in aerosol spray products. There were no CFC’s in the atmosphere before 1930.
Earth’s average surface temperature has risen sharply since the mid-1900’s. Most scientists believe the greenhouse effect is responsible for most of this global warming. In the greenhouse effect, certain gases in the atmosphere trap heat from the sun, acting much like the glass roof and walls of a greenhouse. The chief greenhouse gases include methane and carbon dioxide. Modern industry has caused significant increases in greenhouse gases. Many scientists believe that CFC’s also strengthen the greenhouse effect.
CFC’s also weaken Earth’s protective layer of ozone. When CFC’s drift high in the atmosphere, they break apart and release chlorine atoms. The chlorine reacts with the ozone, converting it into ordinary oxygen molecules. This conversion enables an increased amount of harmful ultraviolet radiation to reach Earth’s surface. By 2000, the United States and most other industrialized countries had ended production of CFC’s.
The study of air
Since ancient times, people have known that air is important to life. During the 400’s B.C., Empedocles, a Greek philosopher, suggested that four elements—air, earth, fire, and water—combined in various proportions to make up all objects in the universe. Many other Greek scholars accepted this theory. In the 300’s B.C., the Greek philosopher Aristotle wrote Meteorologica. This book contained his thoughts on the earth sciences, including his theories about the nature of air and weather.
The early philosophers and scientists could not test their theories about the air because they had no instruments to measure the air’s properties. Around 1600, scientists began to use a type of thermometer to study air. Evangelista Torricelli, an Italian mathematician and physicist, invented the mercury barometer in 1643. In the mid-1600’s, the Irish chemist Robert Boyle used the barometer to formulate the relationship between the volume of air and its pressure.
In the 1700’s, scientists began to study the gases that make up air. Oxygen was discovered by the Swedish chemist Carl Scheele in the early 1770’s and independently by the English chemist Joseph Priestley in 1774. In 1777, Antoine Lavoisier, a French chemist, realized that oxygen in the air enables objects to burn. Daniel Rutherford, a Scottish physician, discovered nitrogen in 1772. In 1894, the Scottish chemist Sir William Ramsay and the English physicist Lord Rayleigh together isolated argon.
During the early 1900’s, Norwegian researchers headed by the physicist Vilhelm Bjerknes discovered that the movement of enormous bodies of air, called air masses, helps determine weather conditions. The researchers used the term front to signify the location where two different air masses meet each other. They found that storms develop along fronts. Their model of weather systems improved the accuracy of weather forecasting.
Today, weather balloons, radars, satellites, and other devices monitor atmospheric conditions, air pollution levels, and changes in the composition of the air. Meteorologists can analyze the data to prepare detailed weather forecasts and to study Earth’s climate.
In 1999, the National Aeronautics and Space Administration (NASA) launched Terra, the first of a series of satellites known as the Earth Observing System. Terra monitors clouds, water vapor, aerosol particles, and trace gases in the atmosphere linked to global climate change.
