Aluminum << uh LOO muh nuhm >> is a lightweight, silver-colored metal that can be formed into almost any shape. It can be rolled into thick plates for armored tanks or into thin foil for chewing gum wrappers. It may be drawn into wire or made into cans. Aluminum does not rust, and it resists wear from weather and chemicals. Aluminum is called aluminium << `al` yuh MIHN ee uhm >> in English-speaking countries outside North America.
Pure aluminum is soft and has little strength. For this reason, aluminum producers almost always alloy (mix) it with small amounts of copper, magnesium, zinc, and other elements to form aluminum alloys. The added elements give aluminum strength and other properties that make it one of the most useful metals. The world uses more aluminum than any other metal except iron and steel.
The largest share of aluminum alloy production goes to the packaging industry for use in such items as beverage cans, bottle caps, foil pouches, foil wrappers, and food containers. The construction industry uses aluminum alloys in such items as gutters, panels, residential siding, roofing, tubes for electric wires, and window frames. Manufacturers of transportation equipment use huge amounts of aluminum in airplanes, automobiles, boats, railroad cars, and trucks. Aluminum is used in much electrical equipment, including light bulbs, power lines, and telephone wires. Thousands of other products also contain aluminum. These products include air conditioners, cookware, golf clubs, knitting needles, lawn furniture, license plates, paints, refrigerators, rocket fuel, and zippers.
Aluminum is the most plentiful metallic element in Earth’s crust and the third most common of all the elements, after oxygen and silicon. Aluminum makes up about 8 percent of Earth’s crust. But unlike some other metals, such as gold and silver, aluminum metal never occurs free (uncombined) in nature. It is always chemically combined with other elements in minerals. People had no way of separating aluminum from these elements until the 1800’s. Scientists then developed processes for separating the minerals and producing aluminum. These processes have been used to make aluminum ever since.
See Element, Chemical (table: Table of the elements).
Properties of aluminum alloys
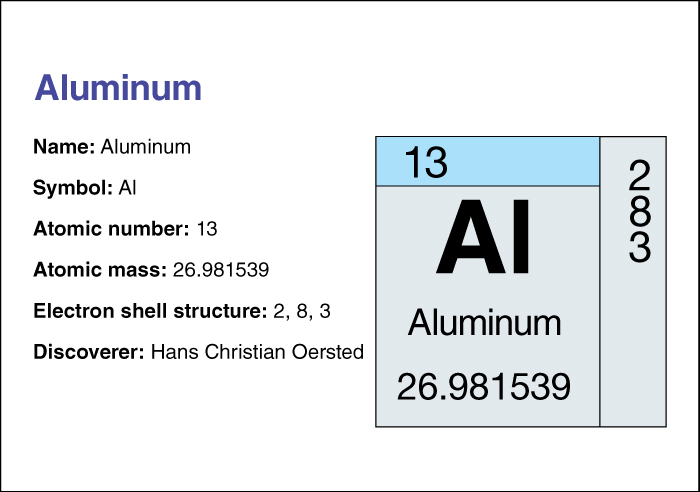
The chemical symbol for aluminum is Al. Aluminum has an atomic number (number of protons in its nucleus) of 13. Its relative atomic mass is 26.981538. An element’s relative atomic mass equals its mass (amount of matter) divided by 1/12 of the mass of carbon 12, the most abundant form of carbon. Aluminum melts at 660.2 °C and boils at 2500 °C. At 20 °C, it has a density of about 2.70 grams per cubic centimeter (see Density). Chemists classify aluminum as an other metal. For information on the position of aluminum on the periodic table, see the article Periodic table.
Only a small percentage of aluminum is used in pure form. It is made into such items as electrical conductors, jewelry, and decorative trim for appliances and cars.
Almost all aluminum is used commercially in alloy form with up to 15 percent of one or more other elements. The chief elements are copper, magnesium, manganese, silicon, tin, and zinc. Copper and magnesium increase the strength and hardness of aluminum. Magnesium also makes aluminum easier to weld. Manganese helps aluminum resist corrosion and also provides strength. Silicon lowers the melting point of aluminum and makes it easier to cast. Tin makes aluminum easier to shape with metalworking tools. Zinc, especially when combined with magnesium, gives added strength. Other elements may also be alloyed with aluminum for special purposes. These elements include bismuth, boron, cadmium, chromium, cobalt, iron, lead, lithium, nickel, sodium, titanium, vanadium, and zirconium.
Aluminum, with its alloys, has many valuable properties that make it an exceptionally useful metal. These properties include (1) light weight, (2) strength, (3) corrosion resistance, (4) electrical conduction, (5) heat conduction, and (6) light and heat reflection.
Light weight.
Aluminum is one of the lightest metals. It weighs about 170 pounds per cubic foot (2,720 kilograms per cubic meter)—about a third as much as steel. As a result, aluminum has replaced steel for many uses. For example, some parts of airplanes, automobiles, and trucks are now made of aluminum rather than of steel because lighter vehicles use less fuel. Products packed in aluminum containers cost less to ship because the containers weigh less than those made of other metals. To make aluminum alloys even lighter, the lightest metal, lithium, is added to the aluminum.
Strength.
Although pure aluminum is weak, certain aluminum alloys are as strong as steel. Such alloys are used in airplanes and trucks, in guardrails along highways, and in other products that require strength. Aluminum alloys lose some strength at high temperatures. But unlike many other metals, they get stronger at extremely low temperatures. Aluminum alloys are widely used in equipment for processing, transporting, and storing liquefied natural gas, which can have a temperature of 2260 °F (2162 °C).
Corrosion resistance.
Some metals corrode (wear away) if exposed to oxygen, water, or various chemicals. A chemical reaction occurs that causes the metals to rust or become discolored. When aluminum reacts with oxygen, however, the metal forms an invisible layer of a chemical compound called aluminum oxide. This layer protects aluminum from corrosion by oxygen, water, and many chemicals. It makes aluminum especially valuable for use outdoors, where the metal resists the effects of wind, rain, and pollution.
Electrical conduction.
Aluminum and copper are the only common metals suitable for use as electrical conductors. Aluminum conducts electric current about 62 percent as well as copper. But aluminum weighs a third as much. Aluminum wire can therefore carry the same amount of electric power as copper wire that weighs twice as much. In addition, aluminum is more ductile than copper, which means it can more easily be drawn into wires. Aluminum wire reinforced with steel is used for nearly all high-voltage power lines.
Heat conduction.
The first large commercial use of aluminum was in cookware. Aluminum cookware heats quickly and evenly. Aluminum also cools quickly, which helps make it popular for such items as beverage cans and radiators.
Light and heat reflection.
Aluminum reflects about 80 percent of the light that strikes it. Because of this property, aluminum is widely used in lighting fixtures. Aluminum also reflects heat well. Buildings with aluminum roofs reflect much of the sun’s heat and so stay cooler in hot weather. When firefighters must walk through flames, they wear special suits coated with aluminum.
Other properties.
Aluminum is nonmagnetic, which makes it valuable for protecting electrical equipment from magnetic interference. Aluminum does not produce sparks when struck and so can be used near flammable or explosive materials. The metal is not poisonous, and so food can be safely wrapped in aluminum foil and cooked in aluminum pots. Aluminum can be shaped by almost any metalworking process. It can also be bolted, glued, riveted, soldered, welded, and otherwise joined by most methods used for other metals. Finally, aluminum can be recycled.
Sources of aluminum
Most minerals, rocks, and soils contain aluminum compounds. But aluminum can be made economically only from bauxite. Bauxite is the name for any ore that has a large amount of aluminum hydroxide—a chemical combination of aluminum oxide and water. Aluminum oxide, also called alumina, is the compound from which aluminum is made.
Most bauxite consists of 30 to 60 percent alumina and 12 to 30 percent water. It also contains iron oxide, silica, and titanium oxide. The color of bauxite depends chiefly on how much iron oxide the ore contains. The more iron oxide it has, the darker the color. Bauxite may be white, cream, gray, pink, yellow, red, or brown. Most bauxite is as hard as rock, but some is as soft as clay.
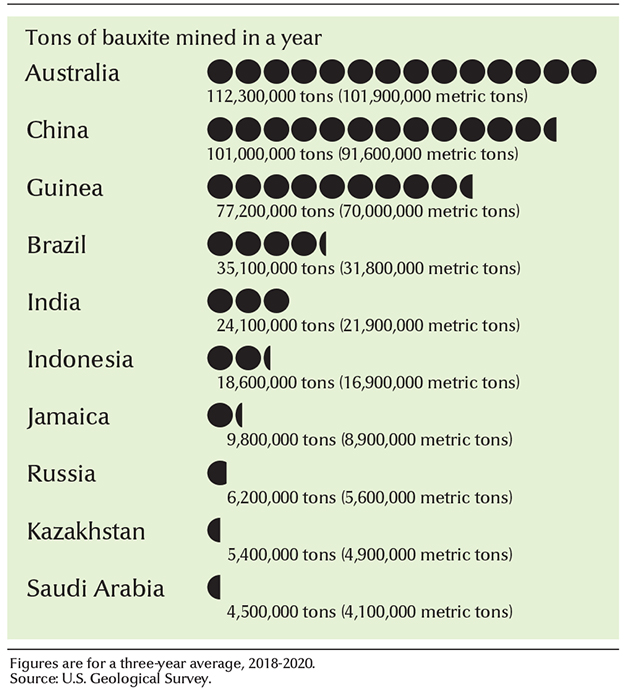
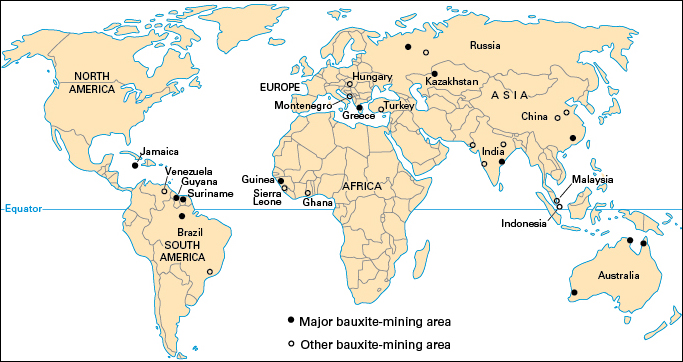
The richest deposits of bauxite lie in tropical and near-tropical regions. Enough bauxite deposits have been found to last several hundred years. The leading bauxite-mining countries include Australia, Brazil, China, Guinea, India, Indonesia, and Jamaica.
Most bauxite deposits lie near the surface of Earth and are mined by the open-pit method, also called the opencast method. In this process, bulldozers and other earthmoving machines first clear away the overburden—the soil, rocks, and trees that cover the deposits. Next, explosives are used to blast the ore loose. Huge power shovels scoop up the bauxite, and trucks or railroad cars carry it to a processing plant.
At the processing plant, the bauxite is crushed and then washed to remove clay and dirt. Some of the water in the bauxite is removed by drying the ore in kilns (ovens). The bauxite is then ground into a powder and shipped to a refining plant.
How aluminum is produced
There are two chief steps in producing aluminum: (1) refining the bauxite to obtain alumina and (2) smelting the alumina to obtain aluminum. After smelting, the molten aluminum is cast into blocks called ingots or other forms that will be shaped into finished products. It takes 4 to 6 pounds (1.8 to 2.7 kilograms) of bauxite to make 1 pound (0.5 kilogram) of aluminum.
Refining the bauxite
separates the alumina in the ore from the iron oxide, silica, and titanium oxide. To separate the alumina, aluminum producers use the Bayer process. This technique was patented by Karl Joseph Bayer, an Austrian chemist, in 1888.
The first step of the Bayer process is mixing powdered bauxite with a solution of sodium hydroxide (also called caustic soda, or lye). Machines pump the mixture into large tanks called digesters. The digesters heat the mixture under pressure at 300 to 480 °F (150 to 250 °C) for about 30 minutes. The alumina dissolves in the sodium hydroxide solution, forming a solution of sodium aluminate. The other materials in the bauxite remain as solids and are called red mud because of their color.
The mixture of sodium aluminate solution and red mud next passes through a series of tanks in which cloth filters separate the liquid from the solids. The red mud is discarded. The sodium aluminate solution is cooled slightly and sent to tanks called precipitators. Crystals of aluminum hydroxide are then added to the solution, which is agitated (stirred) for several days. This process causes most of the alumina in the solution to precipitate (come out of solution) and collect on the crystals.
After the alumina has precipitated, the solution is filtered to separate the aluminum hydroxide crystals from the liquid. The crystals are washed to remove any impurities and then heated at 2000 to 2200 °F (1090 to 1200 °C). The heat drives out water, leaving a fine white powder of alumina. The alumina is composed of aluminum and oxygen. To recover the alumina that did not precipitate, manufacturers take the liquid and refine it with a new batch of bauxite and sodium hydroxide. Small amounts of lime and soda ash may also be added.
Smelting the alumina
separates the aluminum in the powder from the oxygen. The smelting is done by the Hall-Heroult process. Two scientists, Charles Martin Hall of the United States and Paul L. T. Heroult of France, developed this method independently in 1886.
Aluminum producers begin the Hall-Heroult process by dissolving the alumina in a chemical bath composed mainly of cryolite (sodium aluminum fluoride). The bath also contains a little aluminum fluoride and calcium fluoride. The bath is held in large rectangular steel containers and heated to about 1740 °F (950 °C). The containers, called pots or cells, have a carbon lining.
In a process called electrolytic reduction, one or more carbon blocks suspended in each pot send an electric current through the bath. The current flows to the carbon lining, completing the electric circuit. The blocks act as the anode, or positive pole of the circuit, and the lining acts as the cathode, or negative pole. As the current flows through the bath, the alumina breaks apart. The oxygen in the alumina combines with the carbon in the anode and is released as carbon dioxide gas. The aluminum metal collects at the cathode at the bottom of the pot. See Electrolysis.
An aluminum plant may have as many as 200 pots electrically connected to one another in long rows called potlines. The reduction of alumina to aluminum goes on continuously. Alumina is added to the pots regularly, and the electric current keeps the bath at the proper temperature. A large pot may produce more than 2 tons (1.8 metric tons) of aluminum daily.
Casting the molten aluminum.
About once a day, molten aluminum from the potlines is drawn off into pots called crucibles. Each crucible holds 4,000 to 8,000 pounds (1,800 to 3,600 kilograms) of aluminum. Most of the aluminum is cast into ingots. There are two types of ingots: (1) fabricating ingots and (2) foundry ingots. Aluminum is also cast into forms called billets and into thin slabs.
Fabricating ingots,
or rolling ingots, are used by aluminum producers to make plates, sheets, and foil. The ingots may be 30 feet (9 meters) long, 6 feet (1.8 meters) wide, and 2 feet (0.6 meter) thick. They may weigh up to 18 tons (16 metric tons).
To make fabricating ingots, producers alloy other metals with the molten aluminum in a furnace and then purify the mixture. Scrap aluminum and recycled aluminum may also be added. The purification process, called fluxing, usually consists of pumping chlorine, or a mixture of chlorine with argon and nitrogen, through the liquid. The gas causes impurities to float to the surface, where they are skimmed off. Water vapor in the air reacts with aluminum metal to produce hydrogen gas along with oxides and hydroxides of aluminum. Some of this hydrogen gas becomes trapped in the molten aluminum. Degassing involves the addition of one or more gases to the liquid to remove the hydrogen. Gases used in this process include argon, chlorine, and nitrogen, either alone or in various combinations.
After fluxing and degassing, the molten aluminum alloy is filtered to remove solid impurities. It is then cast into ingots, usually by the direct chill method, also known as direct chill casting. In this process, the alloy is poured into a cooled mold. The mold helps solidify the outside of the ingot, but the core remains molten. The ingot then passes through a spray of cold water. The water quickly cools and hardens the entire alloy.
Foundry ingots,
also called alloy ingots or remelt ingots, weigh 4 to 50 pounds (1.8 to 23 kilograms). In most cases, the molten aluminum is poured from the crucibles directly into molds, where it cools and hardens gradually. Aluminum producers sell foundry ingots to plants called foundries. The foundries remelt the ingots with scrap and recycled aluminum and perform the alloying, fluxing, and degassing operations themselves. The alloyed aluminum is then recast and turned into parts for appliances, automobiles, and other products. Aluminum producers supply some foundry ingots in alloy form. Foundries near aluminum plants may buy molten aluminum that comes directly from the potlines to eliminate the need for remelting.
Billets
are made either in long rectangular shapes that look like railroad ties or in the shape of logs. They are produced in the same way as fabricating ingots. Billets can be made into bars, rods, and parts for thousands of items. Bars look like small rectangular billets. Bars may also be hexagonal or octagonal. Rods look like small pole-shaped billets. Bars and rods are made into tubing, wire, and various other products.
Thin slabs
are made by roll casting—that is, by feeding molten aluminum alloy between two cooled rolls. The aluminum solidifies into a slab less than 1 inch (2.5 centimeters) thick. Roll-cast slabs are then further rolled to produce aluminum plates, sheets, strips, or foil.
How aluminum is shaped and finished
Aluminum ingots and billets can be shaped by any of the metalworking processes. These processes include (1) rolling, (2) casting, (3) extruding, (4) drawing, (5) deep drawing, (6) forging, and (7) machining. After the aluminum is shaped, various finishes may be applied.
Rolling
consists of reducing the thickness of fabricating ingots by squeezing them between pairs of heavy rollers. The ingots are heated and then rolled to a thickness of 1 to 3 inches (2.5 to 7.6 centimeters). After cooling, the metal is rolled again to form plates, sheets, or foil. Aluminum plates are 1/4 inch (6.4 millimeters) thick or more. They are used in applications in which heavy construction is required, such as in railroad cars, ships, and storage tanks. Aluminum sheets measure 6/1,000 to 1/4 inch (0.15 to 6.4 millimeters) thick. They are used for the “skins” of airplanes and in such products as awnings and body panels for motor vehicles. Aluminum foil is less than 6/1,000 inch (0.15 millimeter) thick. It has many household uses, especially in cooking and in wrapping food. Rolling may be used to shape aluminum billets into bars and rods.
Casting
is a process in which alloyed foundry ingots are melted and then poured or forced into molds of a desired shape. The aluminum is removed from the molds after it hardens. Casting is used to make parts of particular items, such as the bottoms of electric irons or parts for automobile engines. See Cast and casting.
Extruding
consists of forcing a heated billet through an opening in a tool called a die. A ram at one end of a cylinder forces the billet through a die opening at the other end. The aluminum comes out shaped like the die opening. The extrusion process is used to make rods and tubing, trim for automobiles, and frames for doors and windows. See Extrusion.
Drawing
produces aluminum wire and tubing. To make wire, a pointed aluminum rod is pulled through a series of successively smaller dies. The rod becomes wire when it reaches a diameter of less than 3/8 inch (9.5 millimeters). Tubing is made by pulling an aluminum rod through one die. A steel bar called a mandrel extends through the center of the die and hollows out the rod.
Deep drawing
forms aluminum into beverage cans, beer barrels, pots and pans, and various other containers. In this process, a ram forces aluminum plate or an aluminum sheet into a cavity of the desired shape.
Forging
is the process of hammering or pressing heated aluminum ingots or billets into the desired shape. Forging produces exceptionally strong parts for use in aircraft landing gear, truck wheels, tools, and various other items. See Forging.
Machining.
Aluminum can be shaped with a variety of machine tools, including drills, grinders, saws, and shears. Such tools shape aluminum bars and rods into bolts, screws, and other small items. Machining may also be used to put final touches on products that have been cast or forged. See Machine tool.
Other shaping processes
produce aluminum in such forms as powders and pastes, which consist of finely ground particles of aluminum. Aluminum powder goes into such products as explosives and inks. In paste form, aluminum is used in paints and in metallic finishes for automobiles.
Aluminum powder is also used to produce gears and other small parts by a process called powder metallurgy. In this process, the aluminum powder is pressed into the desired shape and then heated to bond the particles together. Powders of other metals also may be mixed with the aluminum. Powders of aluminum alloys also may be used. The item is further shaped by forging or some other process. See Powder metallurgy.
Finishing aluminum.
Aluminum has an attractive natural appearance and so is often used without a special finish. However, various finishes may be used for decoration or to improve resistance to corrosion and wear. More kinds of finishes can be applied to aluminum than to any other metal. There are four types of finishes. They are (1) mechanical, (2) chemical, (3) electrochemical, and (4) applied.
Mechanical finishes
include such processes as embossing and polishing. In embossing, a raised pattern is made on aluminum sheets by passing them between rollers that have been engraved with a design. A method called barrel burnishing polishes aluminum articles in a revolving or vibrating barrel that contains an abrasive (gritty) substance.
Chemical finishes
include acid and alkaline etches, which eat designs into aluminum. Acid etches are also used to remove stains from the metal and to prepare it for further finishing. Alkaline etches may be used to give aluminum a dull finish.
Electrochemical finishes
include anodizing and electroplating. Anodizing thickens aluminum’s natural coating of aluminum oxide and thus increases resistance to corrosion, scratching, and wear. It also makes aluminum easy to dye. Electroplating involves coating aluminum with another metal. Certain coatings improve aluminum’s corrosion resistance, electrical conduction, or other properties. See Anodizing; Electroplating.
Applied finishes
include such coatings as enamel, lacquer, paint, and plastic film. They may be applied by dipping, spraying, rolling, or other methods.
The aluminum industry
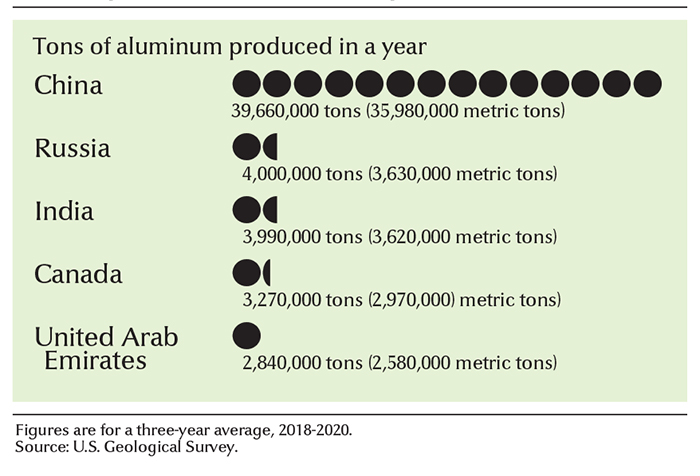
About 40 countries produce aluminum. The world’s annual aluminum production totals about 72 million tons (65 million metric tons). China is the leading aluminum producer, accounting for about half of the world total.
The primary aluminum industry consists of companies that produce aluminum ingots and billets and shape them into such forms as plates, sheets, foil, bars, rods, and wires. The firms also sell the ingots and billets to foundries in the secondary aluminum industry, which specializes in shaping aluminum. Specialized workers in the primary aluminum industry include engineers, geologists, and metallurgists.
In China,
the primary aluminum industry produces about 40 million tons (36 million metric tons) of metal a year. Aluminum Corporation of China Limited (Chalco) is the country’s leading aluminum producer.
In Russia,
the primary aluminum industry produces about 4 million tons (3.6 million metric tons) of metal annually. The country’s leading aluminum producer is United Company Rusal, formed by the merger of the Russian Aluminum Company (Rusal), the Siberian Ural Aluminum Company (SUAL), and Glencore International AG.
In the United States,
the primary aluminum industry produces about 1.1 million tons (1 million metric tons) of the metal yearly. The leading aluminum companies in the country are Alcoa Corporation and Century Aluminum Company.
Aluminum producers in the United States import almost all of their bauxite, chiefly from Brazil and Jamaica. Aluminum producers also import alumina from such countries as Australia, Brazil, and Jamaica.
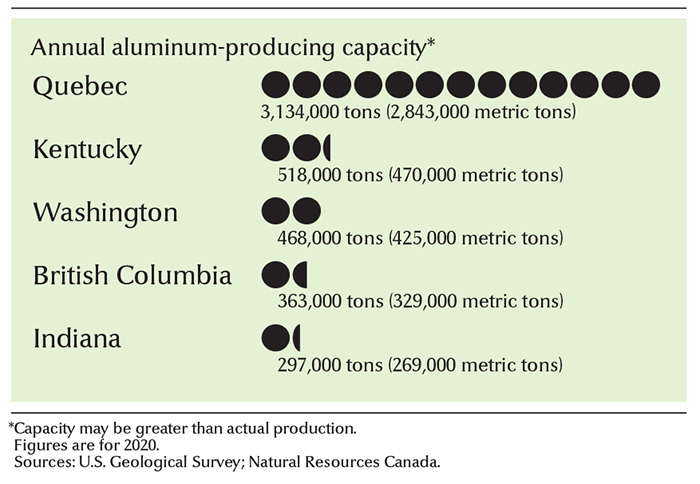
In Canada,
the primary aluminum industry produces about 3 1/4 million tons (3 million metric tons) of the metal yearly. The vast majority of Canada’s aluminum is made in Quebec. The country’s leading aluminum manufacturer is Rio Tinto Alcan. Alcoa Corporation, based in the United States, also produces a significant amount of aluminum in Canada. Canada produces no bauxite. It imports most of this ore from Australia, Guinea, Guyana, and Suriname.
In other countries.
Only about a third of the aluminum-producing countries perform each step in aluminum production—mining the bauxite, refining the ore, and smelting the alumina. In some countries, such as Guinea and Jamaica, the industry mines and refines bauxite for export but produces no aluminum. Germany and some other countries import bauxite and then refine it and smelt the alumina. Some countries, including Norway and Tajikistan, import alumina but not bauxite. Countries that perform each step in the production process include Australia, Brazil, and Suriname.
Recycling
Recycling has become an important aspect of the aluminum industry in many countries. Heavily recycled items include used beverage cans, parts from old automobiles, and scrap accumulated during the manufacture of aluminum products.
Loading the player...Aluminum recycling
One benefit of recycling aluminum is that it conserves natural resources. The most important natural resource saved is energy. Recycling saves about 95 percent of the energy required to make aluminum from bauxite. One recycled aluminum can saves enough energy to keep a 100-watt light bulb burning for about 31/2 hours. In addition, recycling reduces the amount of garbage sent to sanitary landfills.
History
The word aluminum comes from the term alumen. Alumen is the Latin name for alum, a group of aluminum compounds that occur in nature and which ancient peoples used in dyeing textiles. In 1746, Johann Heinrich Pott, a Prussian chemist, prepared alumina from alum. Scientists believed that alumina was a chemical compound that consisted of oxygen and an unknown metal. The British chemist Sir Humphry Davy called this metal alumium and later changed the name to aluminum. In 1809, Davy formed an alloy of iron and aluminum by electrically melting alumina with iron and carbon.
The first aluminum.
In 1825, Hans Christian Oersted, a Danish physicist, produced the first aluminum. Oersted prepared aluminum chloride from alumina. He then heated the aluminum chloride with an alloy of potassium and mercury, and a small lump of impure aluminum formed in the alloy.
In 1827, Friedrich Wöhler, a German chemist, produced aluminum in the form of a gray powder by heating aluminum chloride with potassium. In 1845, Wöhler produced aluminum particles large enough to be weighed. He discovered that aluminum was lightweight. Wöhler was the first scientist to describe several other properties of aluminum.
In 1854, Henri Étienne Sainte-Claire Deville, a French chemist, improved on Wöhler’s method. Deville used sodium instead of potassium to break down aluminum chloride. This process produced larger quantities of aluminum. Commercial aluminum plants using Deville’s method soon opened in France. The price of aluminum dropped from $115 (U.S.) a pound ($254 a kilogram) in 1855 to $17 a pound in 1859. However, it was still too costly for widespread use.
Growth of the aluminum industry.
In 1886, two scientists—Charles Martin Hall of the United States and Paul L. T. Héroult of France—developed an inexpensive way to make aluminum. Neither scientist knew that the other was working on the problem. However, each thought of dissolving alumina in the mineral cryolite and separating aluminum from the mixture by electrolytic reduction. Today, the Hall-Héroult process is used to produce nearly all the aluminum in the world.
Karl Joseph Bayer, an Austrian chemist, further reduced the cost of aluminum production. In 1888, he patented an inexpensive method for obtaining alumina from bauxite. The aluminum industry still uses the Bayer process to produce alumina. The Hall-Héroult and Bayer processes are described in the section “How aluminum is produced.”
Hall and several business associates organized the Pittsburgh Reduction Company in 1888. The company began producing 50 pounds (23 kilograms) of aluminum a day. The firm changed its name to the Aluminum Company of America (Alcoa) in 1907. By 1909, Alcoa was producing 16,500 tons (14,970 metric tons) a year. The price of aluminum dropped to less than 30 cents a pound (66 cents a kilogram). Héroult formed a Swiss aluminum company in 1888, but it did not begin production immediately. In 1902, the Northern Aluminium Company, Limited (now Rio Tinto Alcan), was founded in Canada.
Aluminum production soared during World War I (1914-1918) as the fighting nations increased output to help fill their military needs. During the 1920’s, the development of new aluminum alloys and of improved methods of turning aluminum into useful products continued to boost production. The Great Depression of the 1930’s cut world aluminum output almost in half. But the start of World War II (1939-1945) brought tremendous expansion in production.
After World War II, the aluminum industry developed many products that have become commonplace. The first successful aluminum foil wrap appeared in 1947. Also in the 1940’s, aluminum began to replace brass in the base of light bulbs. High-strength aluminum wire for power lines was introduced in 1957. Aluminum cans became popular in the 1960’s. Today, nearly all beverage cans are made completely of aluminum.
Recent developments.
In the late 1900’s, such developing nations as India, Brazil, and China experienced great industrial growth and an increased standard of living. This growth has created new demand for aluminum packaging for foods and beverages, electric transmission cable, automobiles and trucks, and building construction materials.
Increased competition and supply issues have led a number of aluminum countries to join together. In some instances, large companies have bought smaller companies to gain their products and customers. In others, two or more large companies have merged to create even larger corporations. Such corporations can more efficiently supply a wide range of products to customers around the world. Government agencies study merger plans and must give permission for the completion of a merger to avoid creating monopolies.
