Americium, << `am` uh RIHSH ee uhm or `am` uh REES ee uhm, >> is an artificially created radioactive element . Its chemical symbol is Am and its atomic number (number of protons) is 95. Chemists place americium in the actinide group of transuranium elements . For information on the position of americium on the periodic table, see the article Periodic table .
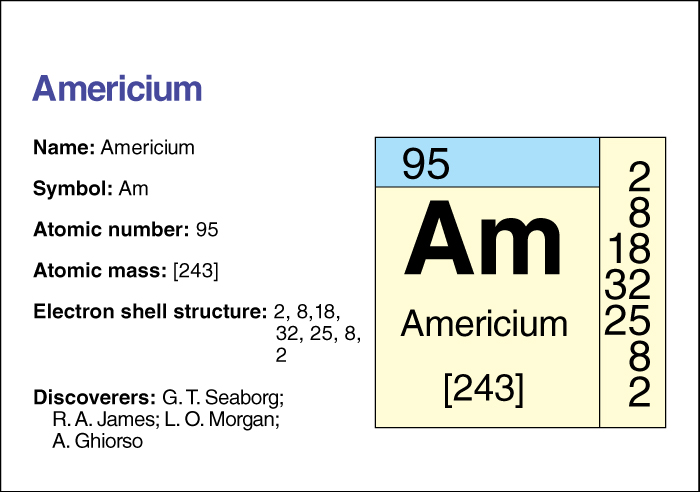
Americium has 15 known isotopes, forms of the element that have the same number of protons but different numbers of neutrons. The most stable isotope has an atomic mass number (total number of protons and neutrons) of 243. That isotope has a half-life of 7,370 years—that is, due to radioactive decay, only half the atoms in a sample of Americium 243 would still be atoms of that isotope after 7,370 years (see Radiation (Radiation and radioactivity) ). Only this isotope and one that has a mass number of 241 and a half-life of 432 years can be produced in large amounts. At 20 °C, americium has a density of 13.67 grams per cubic centimeter (see Density ). The metal melts at 1173 °C. It is an important part of some household smoke detectors, where it gives off so little radiation that it is considered safe to people.
Americium was discovered in 1944 by the American scientists Glenn T. Seaborg, Ralph A. James, Leon O. Morgan, and Albert Ghiorso when they bombarded plutonium with neutrons. It was named for its place of discovery, the United States of America. Americium becomes superconductive (see Superconductivity ) when cooled to –272.36 °C.
See also Isotope ; Seaborg, Glenn T. .
