Atom is one of the basic units of matter. Everything around us is made up of atoms. An atom is incredibly tiny—more than a million times smaller than the thickness of a human hair. The smallest speck that can be seen under an ordinary microscope contains more than 10 billion atoms. The diameter of an atom ranges from about 0.1 to 0.5 nanometer. A nanometer is a billionth of a meter, or about 1/25,400,000 inch.
Atoms form the building blocks of the simplest substances, the chemical elements. Familiar elements include hydrogen, oxygen, iron, and lead. Each element consists of one basic kind of atom. Compounds are more complex substances made of two or more kinds of atoms linked in units called molecules. Water, for example, is a compound in which each molecule consists of two atoms of hydrogen linked to one atom of oxygen.
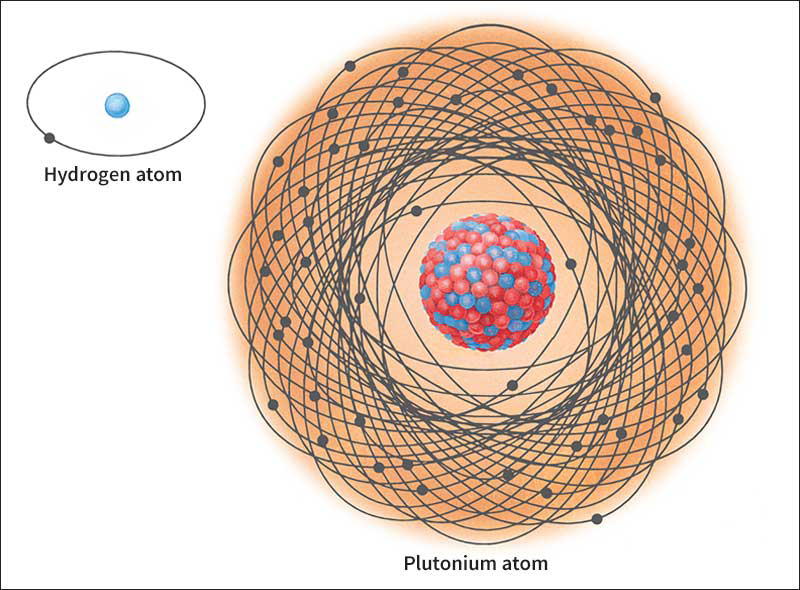
Atoms vary greatly in weight, but they are all about the same size. For example, an atom of plutonium, the heaviest element found in nature, weighs more than 200 times as much as an atom of hydrogen, the lightest known element. However, the diameter of a plutonium atom is only about 3 times that of a hydrogen atom.
The parts of an atom
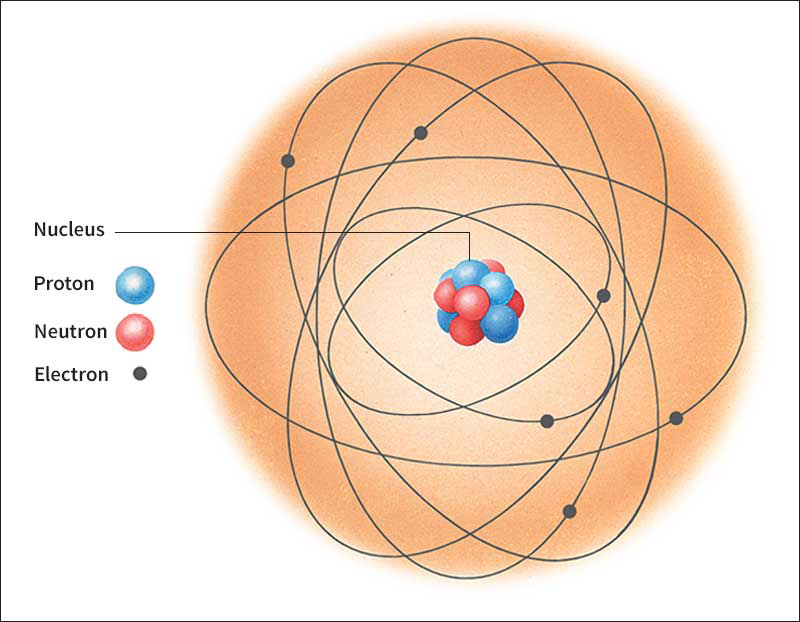
Tiny as atoms are, they consist of even more minute particles. The three basic types are protons, neutrons, and electrons. Each atom has a definite number of these subatomic particles. The protons and neutrons are crowded into the nucleus, an exceedingly tiny region at the center of the atom. If a hydrogen atom were about 4 miles (6.4 kilometers) in diameter, its nucleus would be no bigger than a tennis ball. The rest of an atom outside the nucleus is mostly empty space. The electrons whirl through this space, completing billions of trips around the nucleus each millionth of a second. The fantastic speed of the electrons makes atoms behave as if they were solid, much as the fast-moving blades of a fan prevent a pencil from being pushed through them.
Atoms are often compared to the solar system, with the nucleus corresponding to the sun and the electrons corresponding to the planets that orbit the sun. This comparison is not completely accurate, however. Unlike the planets, the electrons do not follow regular, orderly paths. In addition, the protons and neutrons constantly move about at random inside the nucleus.
The nucleus
makes up nearly all the mass of an atom. Mass is the quantity of matter in an atom. Each proton has a mass roughly equal to that of 1,836 electrons. It would take 1,839 electrons to equal a neutron’s mass. Each proton carries one unit of positive electric charge. Each electron carries one unit of negative charge. Neutrons have no charge. Under most conditions, an atom has the same number of protons and electrons, and so the atom is electrically neutral.
Protons and neutrons are about 100,000 times smaller than atoms, but they are in turn made up of even smaller particles called quarks. Each proton and neutron consists of three quarks. In the laboratory, scientists can cause quarks to combine and form other kinds of subatomic particles besides protons and neutrons. All these other particles break down and change into ordinary particles in a small fraction of a second. Thus, none of them is found in ordinary atoms. However, scientists first learned that protons and neutrons consist of quarks through the study of other subatomic particles. For information on these other particles, see Subatomic particle and the separate articles on subatomic particles listed in the Related articles at the end of this article.
The electrons,
unlike the protons and neutrons, do not seem to have smaller parts. Electrons have almost no mass. The mass of an electron in grams may be written with a decimal point followed by 27 zeros and a 9.
Opposite electric charges attract. The positively charged nucleus therefore exerts a force on the negatively charged electrons that keeps them within the atom. However, each electron has energy and so is able to resist the attraction of the nucleus. The more energy an electron has, the farther from the nucleus it will be. Thus, electrons are arranged in shells at various distances from the nucleus according to how much energy they have. Electrons with the least energy are in inner shells, and those with more energy are in outer shells.
Each electron shell is labeled with a number. The shell closest to the nucleus is called shell 1. The other shells, in order of increasing distance from the nucleus, are shells 2, 3, 4, 5, 6, and 7. The shells are sometimes named by the letters K, L, M, N, O, P, and Q. Each shell can hold only a limited number of electrons. Shell 1 can hold no more than 2 electrons. Shell 2 can hold 8 electrons, shell 3 can hold 18, and shell 4 can hold 32. In theory, shell 5 can hold 50 electrons, shell 6 can hold 72, and shell 7 can hold 98. However, these outer shells are never completely filled.
The properties of atoms
The atomic number
tells how many protons an atom has. All atoms of the same element have the same number of protons. For example, every hydrogen atom has a single proton, and so the atomic number of hydrogen is 1. The atomic numbers for other natural elements range successively up to 92 for uranium, which has 92 protons in each atom. Tiny amounts of plutonium, which has an atomic number of 94, also occur naturally. Elements whose atoms have more than 92 protons can be created in the laboratory.
The atomic number determines an element’s place in the periodic table. This table organizes the elements into groups with similar chemical properties. See Periodic table .
The atomic mass number
is the sum of the protons and neutrons in an atom. Although all atoms of an element have the same number of protons, they may have different numbers of neutrons. Atoms that have the same number of protons but different numbers of neutrons are called isotopes.
Most of the elements in nature have more than one isotope. Hydrogen, for example, has three. In the most common hydrogen isotope, the nucleus consists only of a proton. In the two other hydrogen isotopes, the nucleus consists of one or two neutrons in addition to the proton. Scientists use the mass number to distinguish the three isotopes as hydrogen 1, hydrogen 2, and hydrogen 3. They also refer to hydrogen 1 as protium, to hydrogen 2 as deuterium, and to hydrogen 3 as tritium.
In most lighter elements, the nucleus of each atom contains about an equal number of protons and neutrons. Most heavier elements, however, have more neutrons than protons. The heaviest elements have about 3 neutrons for every 2 protons. For example, uranium 238 has 146 neutrons and 92 protons.
Atoms that have the same mass number but different atomic numbers are called isobars. Thus, isobars are atoms of different elements. For example, the isobars argon and calcium have a mass number of 40, but argon’s atomic number is 18 and calcium’s is 20.
Relative atomic mass
is a measure of how heavy an isotope or an element is. Relative atomic mass is expressed in an unusual way. Measurements of mass are normally given as a number followed by a unit of mass—for example, “18 kilograms.” But relative atomic mass is expressed as a number without a mass unit. For example, the relative atomic mass of the copper 63 isotope, rounded to four digits, is simply “62.93.” The number that represents a relative atomic mass is based on a measurement that consists of a number followed by a unit of mass known as the unified atomic mass unit (u). In fact, the two numbers are identical. Thus, if you know the mass of an isotope or an element in unified atomic mass units, you can express the relative atomic mass by dropping the “u”. For example, the mass of the copper 65 isotope is 64.93 u, and so the relative atomic mass of that isotope is 64.93.
By definition, 1 u equals 1/12 of the mass of an atom of carbon 12. Unified atomic mass units are so tiny that 1 kilogram equals about 6.02 X 1026 u. That number would be written out as 602 followed by 24 zeros.
Calculating the mass of an element.
The mass of an element with only one isotope is simply the mass of that isotope. Scientists determine the mass of an element with more than one isotope by taking into account the proportions in which those isotopes occur in nature. For example, copper has two naturally occurring isotopes: copper 63, with a mass of 62.93 u, represents 69.17 percent of all natural copper; and copper 65, with a mass of 64.93 u, represents the remaining 30.83 percent. The calculation for the mass of the element is (62.93 u X 0.6917) + (64.93 u X 0.3083) = 63.55 u. Dropping the “u” gives the relative atomic mass of the element, 63.55.
Other terms for mass.
In some branches of science, the unified atomic mass unit is called the dalton, represented by the symbol Da. An older term for relative atomic mass is atomic weight.
Electric charge.
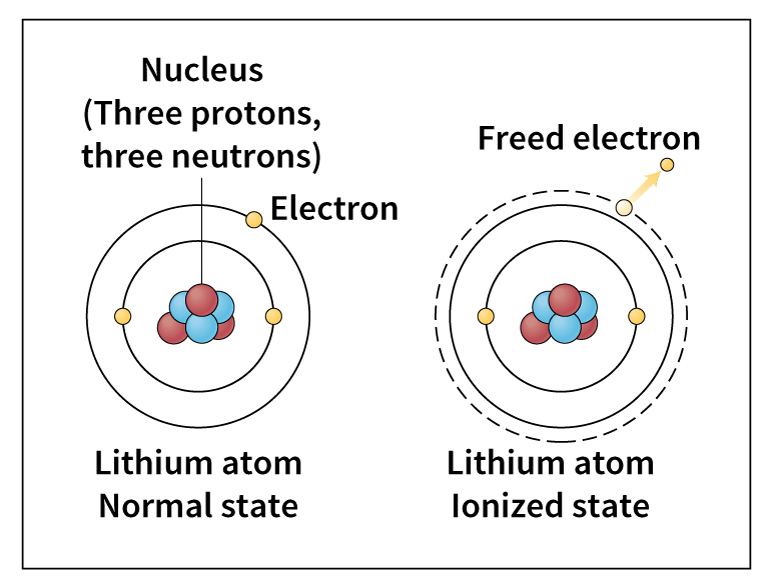
Although an atom is normally electrically neutral, it can lose or gain a few electrons in some chemical reactions, in a collision with an electron or another atom, or when exposed to extremely bright light. This gain or loss of electrons produces an electrically charged atom called an ion. An atom that loses electrons becomes a positive ion, and an atom that gains electrons becomes a negative ion. The gain or loss of electrons is called ionization.
Chemical behavior.
The chemical behavior of an atom is determined largely by the number of electrons in its outermost shell. When atoms combine and form molecules, electrons in the outermost shell are either transferred from one atom to another or shared between atoms. The number of electrons involved in this process is called the valence. The atoms of some elements can have more than one valence, depending on the number and kind of atoms with which they combine.
If an atom tends to lose electrons to other atoms, its valence is positive. If an atom tends to gain electrons, its valence is negative. For example, sodium tends to lose one electron from its outermost shell and thus has a valence of +1. Chlorine tends to accept one electron from another atom and so has a valence of -1. A molecule of ordinary table salt consists of one atom of sodium linked to one atom of chlorine. The sodium atom donates the electron that chlorine is able to accept.
Radioactivity.
In some atoms, the nucleus can change naturally. Such an atom is called radioactive. The change in the nucleus may be only in the arrangement of the protons and neutrons. Or the actual number of protons and neutrons may change. When a nucleus changes, it gives off radiation. This radiation consists of alpha particles, beta particles, or gamma rays. Alpha particles consist of two protons and two neutrons bound together. A beta particle consists of an electron or the oppositely charged positron. Atoms of uranium, radium, and all other elements heavier than bismuth are radioactive. Some isotopes of lighter elements are also radioactive. In addition, physicists can create radioactive isotopes of nearly all elements in a laboratory by bombarding atoms with subatomic particles.
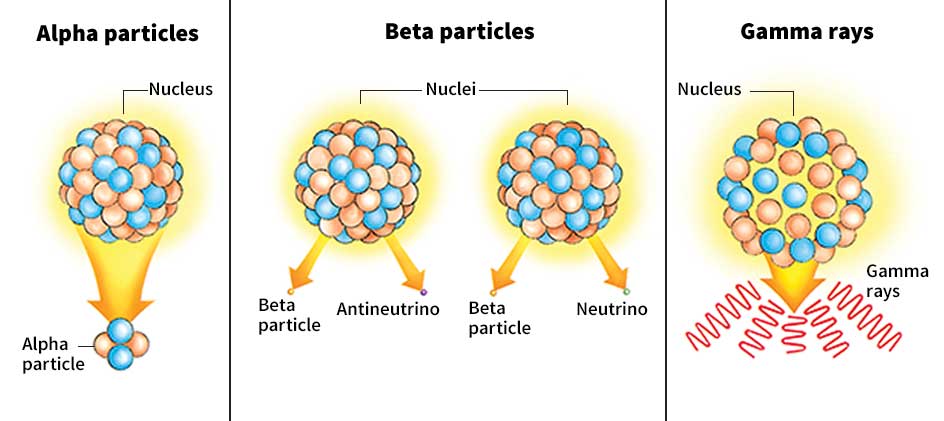
The type of radiation given off by a radioactive nucleus depends on the way the nucleus changes. Gamma rays are given off if only the arrangement of the protons and neutrons in the nucleus changes. But alpha or beta radiation is given off if the number of protons and neutrons in the nucleus changes. The atom then becomes an atom of a different element. This process is called transmutation. See Transmutation of elements.
The forces within an atom
The field of physics called quantum mechanics deals with the forces inside an atom and the motions of subatomic particles. This field began in 1900 when the German physicist Max Planck introduced the idea that radiant energy exists only in specific amounts, later called a quantum or the plural quanta. In 1913, the Danish physicist Niels Bohr used the quantum theory to explain the motion of electrons in atoms. See Quantum mechanics.
Electron energy levels.
According to quantum mechanics, electrons cannot have just any amount of energy. Instead, electrons are restricted to a limited set of motions, each of which has a specific value of energy. These motions are called quantum states or energy levels. When an electron is in a given quantum state, it does not absorb or give off energy. For this reason, an atom can gain or lose energy only if one or more electrons change their quantum state.
Just as water always seeks its lowest possible level, electrons seek the state of lowest energy. However, only one electron at a time can occupy each quantum state. If the lower states are filled, other electrons are forced to occupy higher states. If all electrons are in the lowest possible state, the atom is in its ground state. This condition is normal for atoms at ordinary temperatures.
When matter is heated to temperatures higher than a few hundred degrees, energy is available to raise one or more electrons to a higher energy level. The atom is then in an excited state. However, atoms rarely remain in an excited state for more than a fraction of a second. An excited electron almost immediately drops to a lower state and continues dropping until the atom returns to its ground state. At each succeeding drop, the electron gives off a tiny packet of radiant energy called a photon. The energy of the photon equals the difference between the two energy levels of the electron. The photons given off by electrons are detected as visible light and other forms of electromagnetic radiation.
Bohr originally described the quantum states of electrons as orbits like those of the planets around the sun. However, physicists now know that this description is incorrect because an electron is not simply a particle. An electron also has some characteristics of a wave. It is difficult to imagine how something could be both a particle and a wave. This difficulty is one of the problems scientists have in trying to describe the atom to nonscientists. To do so, scientists must use familiar ideas based on our knowledge of the world as we observe it. But conditions inside the tiny atom differ greatly from those in our everyday world. For this reason, physicists can describe the motions of electrons accurately and completely only in mathematical terms.
Forces in the nucleus.
The quantum rules that govern the motion of electrons also apply to the motion of protons and neutrons inside the nucleus. However, the force that keeps the nuclear particles together differs greatly from the electrical attraction that holds the electrons within the atom. Each nuclear particle is attracted to its nearest neighbor by what is called the strong nuclear force or strong interaction. Like electric charges repel each other. However, the powerful nuclear force overcomes the mutual repulsion of the positively charged protons. It thus keeps the nucleus from flying apart. This force dies off quickly, however, unless the nuclear particles are extremely close together. Electrons are immune to the nuclear force.
The nuclear force is highly complicated, and no exact mathematical description of it has been formulated. Nevertheless, a theory known as the nuclear shell model provides reasonably accurate estimates of the energy levels in the nucleus.
One neutron and one proton can occupy each quantum state in the nucleus. For this reason, a light nucleus has a nearly equal number of protons and neutrons. But a proton and a neutron in the same state do not have the same amount of energy. Each proton is electrically repelled by all other protons in the nucleus, which increases its energy. In a nucleus with many protons, the difference in energy levels between protons and neutrons is considerable, and more low-energy states are available for neutrons than for protons. This is why a heavy nucleus has more neutrons than protons.
How scientists study atoms
Scientists use a variety of instruments and techniques to study atoms. The devices and methods used depend on whether the researchers are studying atoms themselves, electrons, nuclear particles, or quarks.
Researchers use X rays to study the arrangements of atoms in regular, repeated patterns, such as in crystals. When X rays pass through a crystal, the atoms in the crystal diffract (spread out) the X rays in a certain way. These diffracted rays produce patterns on photographic film that reveal how far apart the atoms are and how they are arranged. Extremely powerful scanning electron microscopes, scanning tunneling microscopes, and field-emission microscopes enable scientists to observe the positions of individual atoms. However, these instruments cannot reveal any details of the structure of atoms.

Scientists study the energies of electrons chiefly by analyzing the light given off by atoms in heated gases. Instruments called spectrometers break up the light into a spectrum with a separate line for each wavelength of light. Each wavelength is related to the difference in energy between two quantum states in the atom. After determining the wavelengths, scientists can draw up a complete list of energy levels. With the aid of quantum mechanics, they can then obtain a description of the electron motion in the atom.
Most of what scientists know about nuclear structure has come from experiments with particle accelerators. These devices bombard the nucleus with beams of high-energy electrons or protons. The swift-moving electrons or protons can disrupt the motion of particles in the nucleus and occasionally even knock some of them loose. In some experiments, whole nuclei are accelerated and smashed into stationary nuclei. Nuclear physicists have developed a wide variety of detectors for observing the particles that emerge from these collisions. Most of the detectors produce an electric signal when a particle passes through them.
Particle accelerators are also used to study the behavior of quarks. But such studies require particles with much greater energies than those used to study atomic nuclei. Thus, much more powerful accelerators are required.
Development of the atomic theory
The idea that everything is made up of a few simple parts originated during the 400’s B.C. in the philosophy of atomism. Atomism was founded by the Greek philosopher Leucippus, but his disciple Democritus developed the philosophy more fully. Democritus gave his basic particle the name atom, which means uncuttable. He imagined atoms as small, hard particles, all composed of the same substance but of different sizes and shapes. During the 300’s B.C., a Greek philosopher named Epicurus incorporated Democritus’s ideas about atoms into his philosophy. About 50 B.C., the Roman philosopher and poet Lucretius presented the fundamental principles of atomism in his long poem, On the Nature of Things. See Atomism.
During the Middle Ages, from about the A.D. 400’s through the 1400’s, the idea of atoms was largely ignored. This neglect resulted partly from the fact that atomism had been rejected by Aristotle, an ancient Greek philosopher whose theories dominated medieval philosophy and science. The idea that atoms form the basic units of all matter did survive, however. During the 1500’s and 1600’s, such founders of modern science as Francis Bacon and Isaac Newton of England and Galileo of Italy believed in atoms. But those scientists could add little more to the atomic theory than Democritus had described.
The birth of the modern atomic theory.
In 1750, Rudjer Boscovich, a scientist born in what is now Croatia, suggested that Democritus might have been wrong in believing that atoms are “uncuttable.” Boscovich thought that atoms contain smaller parts, which in turn contain still smaller parts, and so forth down to the fundamental building blocks of matter. He felt that these building blocks must be geometric points with no size at all. Today, most atomic physicists accept a modern form of this idea.

The development of the atomic theory advanced greatly when chemistry became an exact science during the late 1700’s. Chemists discovered that they could combine elements to form compounds only in certain fixed proportions according to mass. In 1803, a British chemist named John Dalton developed an atomic theory to explain this discovery. Dalton proposed that each element consists of a particular kind of atom and that the varying properties of the elements result from differences in their atoms. He further suggested that all atoms of a given element are identical in size, shape, and mass. According to Dalton’s theory, when atoms combine and form a particular compound, they always combine in a specific numerical ratio. As a result, the composition by mass of a particular compound is always the same.
The first descriptions of atomic structure.
In 1897, a British physicist named Joseph John Thomson discovered that atoms are “cuttable.” He made this discovery while studying the rays that travel between charged metal plates in a vacuum tube. Thomson determined that the rays consisted of lightweight, negatively charged particles. He had thus discovered electrons. Thomson immediately realized that the electrons must be part of the atom. He proposed a model of the atom in which negatively charged electrons were embedded in a positively charged sphere. Although Thomson’s description was far from correct, his work encouraged other scientists to investigate the structure of the atom.
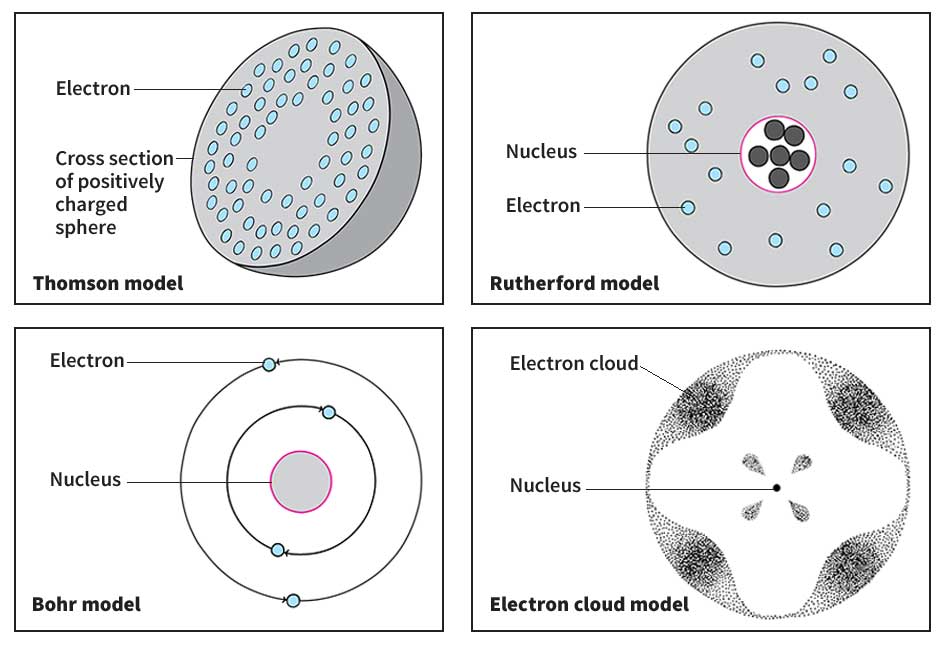
In 1911, the New Zealand-born physicist Ernest Rutherford presented his theory of atomic structure. Rutherford, a former student of Thomson’s, declared that nearly all the mass of an atom is concentrated in a tiny nucleus. He also stated that the nucleus is surrounded by electrons traveling at tremendous speeds through the atom’s outer regions.
Loading the player...Atomic transmutation
Rutherford based his theory on the results of experiments in which he bombarded thin sheets of gold with alpha particles. Most of the particles passed through the sheets, which showed that the gold atoms must consist chiefly of empty space. But some particles bounced back as if they had hit something solid. Rutherford concluded that these particles had been reflected by a strong force from the tiny but heavy nucleus of an atom.
Rutherford’s theory did not explain the arrangement of electrons in atoms. In 1913, however, a description of the electron structure was proposed by Niels Bohr, a Danish physicist who had worked with Rutherford. Bohr suggested that electrons could travel only in a certain set of orbits around the nucleus. Bohr’s original, crude picture of the atom was inadequate, but many of the ideas behind it proved correct. In 1924, the French physicist Louis de Broglie proposed that electrons have some properties of waves. By 1928, a correct description of the arrangement of electrons had been obtained with the help of other physicists, especially Erwin Schrödinger and Wolfgang Pauli of Austria, Max Born and Werner Heisenberg of Germany, and Paul Dirac of England.

Studying the nucleus.
Although physicists understood the motions of electrons by 1928, the nucleus remained largely a mystery. Protons had been identified in 1902, and Rutherford had proposed in 1914 that they must form part of the nucleus. In 1932, a British physicist named James Chadwick discovered that the nucleus also contains uncharged particles, or neutrons. Also in the early 1930’s, scientists developed particle accelerators capable of producing energies high enough to study the nucleus.
The pioneers of nuclear physics did not expect that they would soon see a practical use for their knowledge. In 1938, however, researchers discovered that bombarding the nucleus of a uranium atom with a neutron caused the nucleus to split into two parts and release energy. They called the process nuclear fission. The discovery came a few months before the start of World War II in 1939, and fission was used in atomic bombs that helped end the war in 1945.
The development of atomic weapons made governments aware of the importance of nuclear physics. As a result, they provided great sums of money for nuclear research after the war. The funds made possible the construction of accelerators of increasing size and energy. As these accelerators revealed more and more details of the nucleus, researchers realized that the proton and neutron could not be simple objects. They also found that the neutron did not completely lack an electric charge. Instead, it contained equal amounts of positive and negative charge. In addition, researchers discovered hundreds of new particles. These particles were sufficiently similar to one another and to protons and neutrons to suggest that all nuclear particles might be merely different arrangements of a few simple parts.
Recent discoveries.
By 1964, researchers had turned up enough clues to indicate what the fundamental parts of protons, neutrons, and other nuclear particles might be like. Two California Institute of Technology physicists, the American Murray Gell-Mann and Russian-born George Zweig, thus proposed a theory describing these parts. Gell-Mann named the parts quarks. According to this theory, quarks are always combined in groups of two or three. The original theory required only three kinds of quarks, up (or u), down (or d), and strange (or s), to make up protons, neutrons, and the other particles. But by 1977, experimenters had found not only the u, d, and s, but also a charm (or c) and a bottom (or b) quark.
Physicists concluded that a sixth quark, which they named the top (or t), must also exist. In 1995, scientists at Fermi National Accelerator Laboratory in Batavia, Illinois, announced that they had found the top quark. Physicists are almost certain that there are no more quarks to discover.
Although the basic structure of the atom is well understood, new ways of using atoms continue to be discovered. Atoms are used to make atomic clocks, the most accurate clocks in the world. The most advanced of these clocks are so accurate that they would lose only one second of time over tens of millions of years. Many scientists are also looking to atoms to improve the power and speed of computers. Quantum computers make use of the quantum mechanical properties of atoms or of subatomic particles to quickly perform certain computations. Some such computations might take classical computers thousands or millions of years to complete. Quantum effects are difficult to harness for computing, but researchers continue to create more powerful and stable quantum computer prototypes.
