Barium << BAIR ee uhm >> is a soft, heavy, silver-colored metal element. The metal itself has few uses outside the laboratory. But it combines easily with many other chemicals to form compounds with important industrial uses.
Barium carbonate is used in the manufacture of ceramics and special glass, and to purify certain chemical solutions. It is also an ingredient in clay slurries (watery muds) used in drilling oil wells. Chemical manufacturers often use barium carbonate as a raw material when making other barium compounds. Barium carbonate is poisonous, and so is any barium compound that dissolves in water. Barium titanate is used in sonar detectors and other electrical equipment. Barium nitrate makes signal flares burn with a green flame. Barium ferrite is used to make magnets.
Barium sulfate is an extremely insoluble barium compound that is not poisonous. Doctors use it in X-ray examinations of a patient’s digestive system. The barium sulfate absorbs X rays to show an outline of the intestines on the developed film. Barium sulfate and zinc sulfide form lithopone, a white coloring matter for paint.
Barium is never found in a pure state because it combines so easily with other elements. Pure barium is obtained by passing an electric current through a fused (melted) barium compound, such as barium chloride. A piece of barium metal quickly reacts with oxygen and water vapor in the air to form barium oxide. It must be stored under kerosene to keep it pure.
Barium is found most often as barium sulfate in the mineral barite, or heavy spar. It is also commonly found as barium carbonate in witherite.
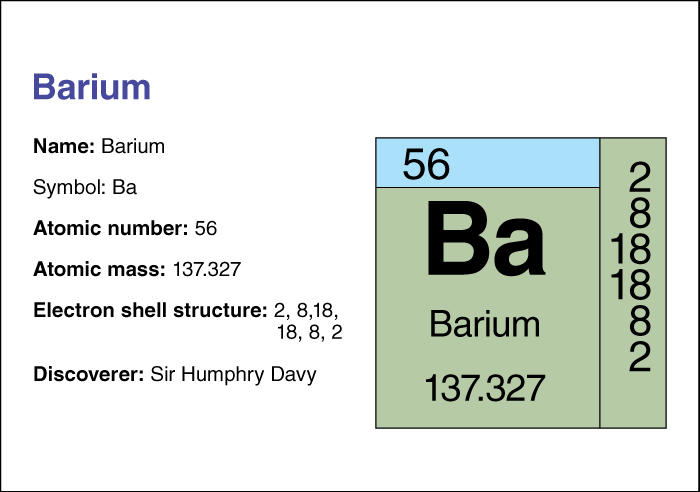
The chemical symbol for barium is Ba.Barium’s atomic number (number of protons in its nucleus) is 56. Its relative atomic mass is 137.327. An element’s relative atomic mass equals its mass (amount of matter) divided by 1/12 of the mass of carbon 12, the most abundant form of carbon. Barium melts at 725 °C (1337 °F) and boils at 1140 °C (2084 °F). Its density is 3.5 grams per cubic centimeter at 20 °C. It was first isolated in 1808 by Sir Humphry Davy, an English chemist.
Chemists classify barium as an alkaline earth metal . For information on the position of barium on the periodic table, see the article Periodic table .
