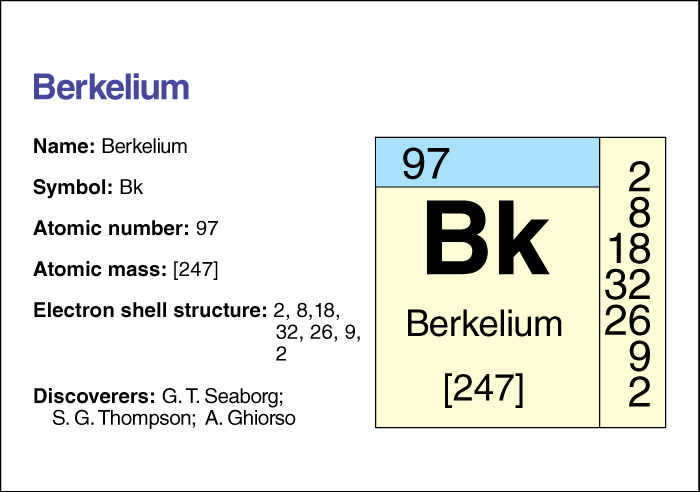
Berkelium << BUR klee uhm or bur KEE lee uhm >> is an artificially created radioactive element. Its chemical symbol is Bk, and its atomic number (number of protons) is 97.
Chemists place berkelium in the actinide group of transuranium elements . For information on the position of berkelium on the periodic table, see the article Periodic table .
Berkelium has 13 known isotopes, forms with the same number of protons but different numbers of neutrons. The most stable isotope has an atomic mass number (total number of protons and neutrons) of 247. That isotope has a half-life of 1,400 years—that is, due to radioactive decay, only half the atoms in a sample of isotope 247 would still be atoms of that isotope after 1,400 years. Isotope 249, which has a half-life of 326 days, can be produced in small amounts in a nuclear reactor. Scientists have used that isotope to study chemical compounds of berkelium.
A team of scientists first prepared and identified berkelium in 1949. They worked at the University of California at Berkeley, after which they named the element. They created berkelium in a machine called a cyclotron. The machine boosted alpha particles, which consist of two protons and two neutrons, to high speeds and then bombarded a sample of the element americium with the particles.
