Californium is an artificially produced radioactive element . Its chemical symbol is Cf, and its atomic number (number of protons in its nucleus) is 98.
Chemists place californium in the actinide group of transuranium elements . For information on the position of californium on the periodic table, see the article Periodic table .
Californium has 20 known isotopes, forms with the same number of protons but different numbers of neutrons. The most stable isotope has an atomic mass number (total number of neutrons and protons) of 251. That isotope has a half-life of 900 years—that is, due to radioactive decay, only half the atoms in a sample of californium 251 would still be atoms of that isotope after 900 years. Another isotope, californium 252, decays partially by nuclear fission, the splitting of the nucleus into two nearly equal parts. During fission, the nucleus releases neutrons and much energy. Because californium 252 can undergo fission, it has found uses in industry and medicine.
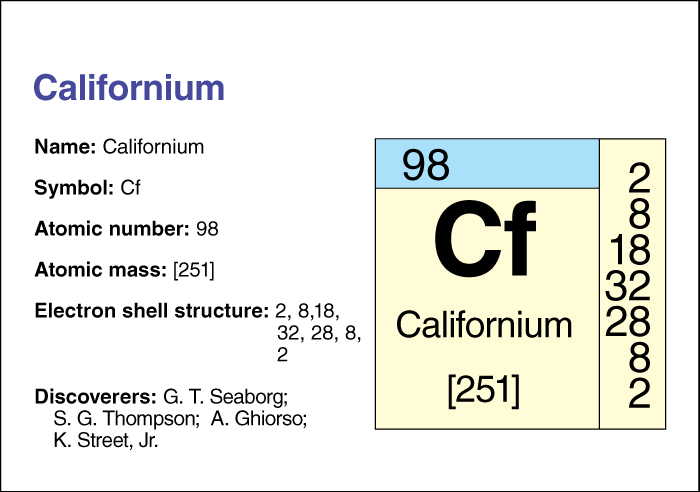
Californium was named after the University of California in Berkeley, where it was discovered in 1950. Four United States scientists—Stanley G. Thompson, Kenneth Street, Jr., Albert Ghiorso, and Glenn T. Seaborg —first produced californium by bombarding the element curium with helium ions (electrically charged atoms). Even though californium is artificially produced, scientists know many of its compounds.
