Electron is a negatively charged subatomic particle. A useful model of an atom portrays the atom as a tiny nucleus surrounded by electrons. The electrons are at various distances from the nucleus and are arranged in energy levels called shells. Electrons occupy almost the entire volume of an atom, but electrons themselves account for only a small fraction of an atom’s mass. The chemical behavior of an atom is determined largely by the number of electrons in its outermost shell. When atoms combine and form molecules, electrons in the outermost shell are either transferred from one atom to another or shared between atoms.
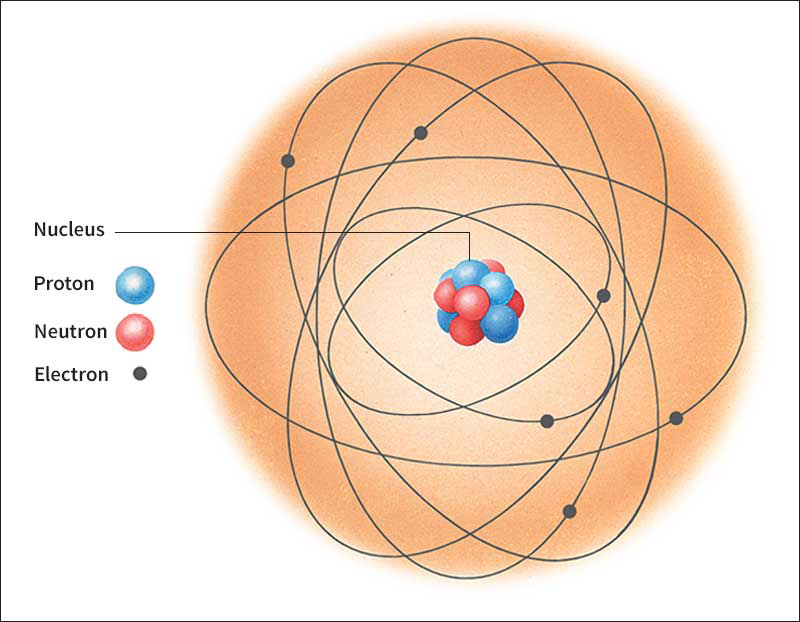
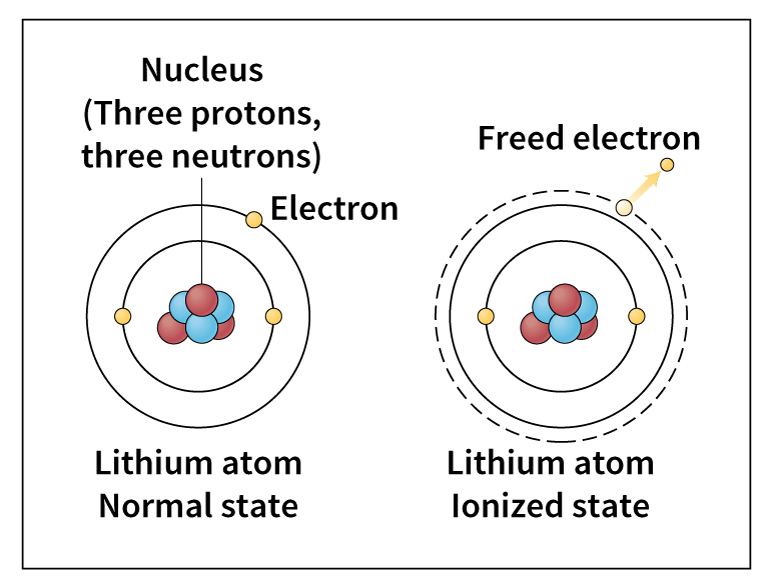
Ordinarily, an atom has an equal number of electrons and protons, positively charged particles found in the nucleus. Each electron carries one unit of negative charge, and each proton carries one unit of positive charge. As a result, the atom is electrically neutral. If an atom gains electrons, it becomes negatively charged. If it loses electrons, it becomes positively charged. Electrically charged atoms are called ions.
Electrons are fundamental units of matter—that is, they are not made up of smaller units. The diameter of an electron is less than 1/1,000 the diameter of a proton. The mass of an electron in grams may be written with a decimal point followed by 27 zeros and a 9. The antimatter equivalent or antiparticle of the electron is the positron. It has a mass equal to that of the electron but a positive charge. Antimatter resembles ordinary matter but with certain properties reversed. Electrons and positrons are the lightest particles that have an electric charge.
The discovery of the electron is generally attributed to Sir Joseph John Thomson, a British physicist who identified it in 1897. In 1913, the American physicist Robert A. Millikan and the Russian physicist Abram F. Ioffe independently reported an accurate measurement of the electron’s charge.
