Europium << yu ROH pee uhm >> (chemical symbol, Eu) is one of the lanthanide metals. It is soft and silver in color.
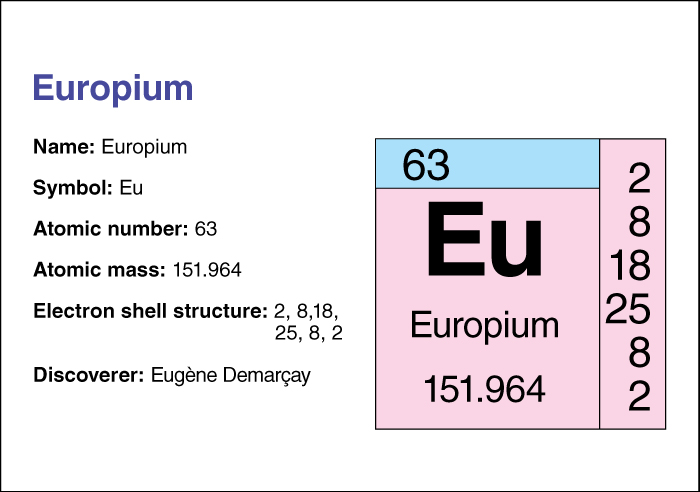
Europium’s atomic number (number of protons in its nucleus) is 63. Its relative atomic mass is 151.964. An element’s relative atomic mass equals its mass (amount of matter) divided by 1/12 of the mass of carbon 12, the most abundant form of carbon. Europium has two naturally occurring isotopes, atoms with the same number of protons but a different number of neutrons. One isotope is stable and one is weakly radioactive. The density of europium is 5.245 grams per cubic centimeter at 25 °C (see Density). For information on the position of europium on the periodic table, see the article Periodic table.
The French scientist Eugene Demarcay first discovered the element in 1901. He named it europium in honor of Europe. Europium occurs in cerium minerals. It melts at 822 °C and boils at 1529 °C. Europium is used in laser technology, alloys (metal mixtures), some fluorescent light bulbs, and nuclear reactor control rods. See also Rare earth.
