Gas is one of our most important resources used as both a fuel and a raw material. We burn gas to provide heat and to produce energy to run machinery. The chemical industry uses the chemicals in gas to make detergents, drugs, plastics, and many other products.

People sometimes confuse gas with gasoline, which is often called simply gas. But gasoline is a liquid. On the other hand, gas fuel—like air and steam—is a gaseous form of matter. That is, it does not occupy a fixed amount of space as liquids and solids do. For information on gas as a form of matter, see the Gas article. See also Gasoline.
Gas has many uses as a fuel. Millions of people use it to heat their homes, cook meals, heat water, dry laundry, and cool the air. Hotels, restaurants, hospitals, schools, and many other businesses and institutions burn gas for cooking, heating buildings and water, cooling the air, and generating steam. Gas produces little air pollution when it is burned.
In addition to using gas as a raw material, industry uses gas flames and gas heat for many applications. These applications range from burning off the quills of chickens to hardening the nose cones of spacecraft.

There are two kinds of gas—natural gas and manufactured gas. Most of the gas used in the world is natural gas. Most scientists believe that natural gas has been forming beneath Earth’s surface for hundreds of millions of years. The natural processes that created gas also created petroleum. As a result, natural gas is often found with or near oil deposits. The same methods are used to explore and drill into Earth for both fuels. Manufactured gas is produced chiefly from coal or petroleum, using heat and chemical processes. Manufactured gas costs more than natural gas and is used in regions where large quantities of the natural fuel are not available.
The gas industry consists of five main activities: (1) exploring for natural gas; (2) producing gas, either by drilling natural gas wells or by manufacturing gas; (3) transmitting gas, usually by pipeline, to large market areas; (4) distributing gas to the user; and (5) storing gas for transmission and distribution at a later time. Each part of the gas industry requires its own special skills and equipment. Some gas companies conduct all five activities, but most companies handle only one or two.
The modern natural gas industry began in the United States. The industry started to expand rapidly in the late 1920’s with the development of improved pipe for transmitting gas economically over great distances. Today, long-distance gas pipelines serve much of the world.
The composition of natural gas
Pure natural gas is made up of chemical compounds of the elements hydrogen and carbon. These compounds are called hydrocarbons. Some hydrocarbons are naturally gaseous, some are liquid, and some are solid. A hydrocarbon’s form depends on the number and arrangement of the hydrogen and carbon atoms in the hydrocarbon molecule. See Hydrocarbon .
Natural gas is composed mainly of methane, the lightest hydrocarbon. In a molecule of methane (CH4), one atom of carbon is bound together with four atoms of hydrogen. Other gaseous hydrocarbons usually found in natural gas include ethane (C2H6), propane (C3H8), and butane (C4H10). Natural gas that is impure may contain such gases as carbon dioxide, helium, and nitrogen. See Butane and propane ; Ethane ; Methane .
When natural gas burns, the hydrocarbon molecules break up into atoms of carbon and hydrogen. The atoms combine with oxygen in the air and form new substances. The carbon and oxygen form carbon dioxide (CO2), an odorless, colorless gas. The hydrogen and oxygen produce water vapor (H2O). As the molecules break up and recombine, heat is released. Heat is measured in Btu’s (British thermal units) in the inch-pound system of measurement customarily used in the United States, and in calories or in joules in the metric system. One cubic foot (28,316 cubic centimeters) of burning gas releases about 1,000 Btu’s, or about 252,000 calories or 1,050 kilojoules. A kilojoule equals 1,000 joules. See British thermal unit ; Calorie ; Joule .
Uses of gas
Gas as a fuel.
About 40 percent of the gas consumed in the industrialized countries is used in residences or by such businesses and institutions as offices, hotels, restaurants, stores, hospitals, and schools. And industry and electric power generation typically consume about 30 percent each. In many less developed countries, most natural gas is used to generate electric power. In both industrialized and less developed countries, small amounts of natural gas are used as transportation fuel and to operate grain dryers, irrigation equipment, and other farm equipment.
For the industrialized countries as a whole, gas provides about 20 percent of the total energy needs. However, many countries of the world lack either large deposits of natural gas or the systems needed to produce and transport it. These countries consume only small amounts of gas, chiefly manufactured gas.
In the home.
Wherever large quantities of natural gas are available, gas is the most popular cooking fuel. One reason for its popularity is that it costs less than most other fuels. In addition, gas can provide the desired amount of heat instantly, be controlled easily and even automatically, and be shut off instantly.
Many residential consumers also use gas to heat their homes and water, burn garbage and trash, dry laundry, and operate air conditioners. Many people cook outdoors on gas grills. Some homes have gas fireplaces or swimming pools that are heated with gas.
Many people who live in mobile homes or in farm areas or other places far from gas pipelines burn liquefied petroleum gas (LPG) for cooking and heating. LPG is also called LP gas, propane, butane, or bottled gas. It is produced from gaseous compounds in petroleum. These compounds become liquid when they are put under pressure. The liquid takes up much less space than the original gas and is easily transported in small pressurized containers. As the fuel is used, normal air pressure changes the liquid back to gas. In some countries, liquefied petroleum gas is used as a fuel for automobiles.
In industry.
Gas has many uses in industry. Companies use gas flames or gas heat in coating, cutting, and shaping metals and other materials. Gas heat is used to harden the nose cones of spacecraft so they do not burn up from the intense heat generated by atmospheric friction. Meat packers use gas flames to remove the bristles from hogs. Manufacturers use gas to produce or process brick and tile, cement, ceramics, glass, foods, iron and steel, paper, textiles, and countless other products. Gas produces heat for industrial processes ranging from about 350 °F (177 °C) for baking automobile finishes to about 3000 °F (1600 °C) for making steel.
Many modern factories have complete heat and power (CHP) systems, which supply all their power needs. In such systems, gas is the only outside source of energy. It powers a turbine or engine that drives a generator to produce electric power. The exhaust heat from the turbine or engine is used for heating and cooling. 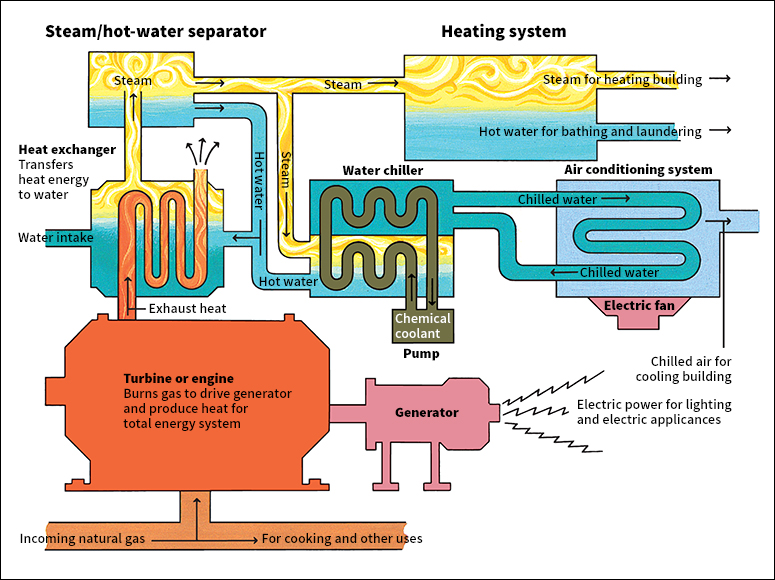
Industry also uses gas infrared heaters. Infrared rays from such heaters heat only the objects that they strike, not the air. These heaters are especially useful for keeping people warm in large warehouses or other buildings that are difficult to heat. See Infrared rays.
Gas as a raw material.
Natural gas is an important source of petrochemicals (chemicals made from natural gas or petroleum). Petrochemicals serve as building blocks for the manufacture of many products, including fertilizers, paints, plastics, and synthetic rubber.
Petrochemical production is based on the various compounds of hydrogen and carbon found in crude gas and oil. These compounds include methane, ethane, and propane. They can be removed from the raw material and used alone, or they can be broken apart and restructured to produce compounds that are not present in the raw material. The compounds or their parts are combined with other chemicals in making detergents, drugs, and other products.
Some components (parts) of natural gas are converted into liquid form, called natural gas liquids (NGL). This group of chemical compounds includes ethane, propane, and butane. For many years, gas producers have made NGL’s by trapping gas in its natural state and storing it at extremely low temperatures or by pressurizing gases that are formed early in the process of refining crude oil. Modern gas-to-liquid (GTL) technologies use chemical processes to convert natural gas to liquids. These technologies can produce NGL’s at lower cost than the traditional methods can. Many NGL plants and petrochemical factories operate near gas fields to be close to their sources of supply. For additional information about the chemistry of gas, see the section The composition of natural gas in this article. See also Natural gas liquids ; Petrochemicals.
How natural gas was formed
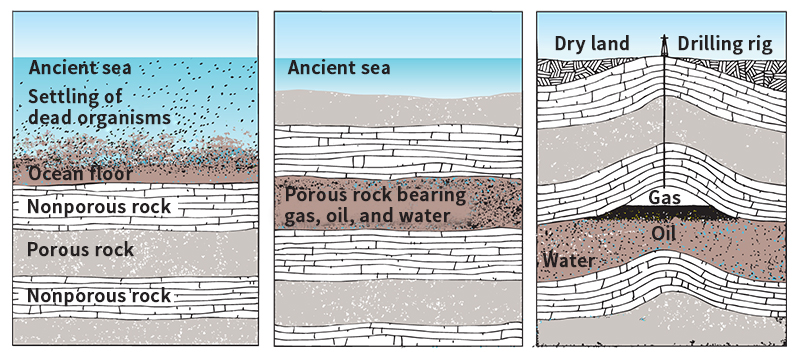
Most scientists believe that natural gas was formed millions of years ago, when water covered much more of Earth’s surface than it does today. Down through the ages, tiny marine organisms called plankton died and settled on the ocean floors (see Plankton ). There, fine sand and mud drifted down over the plankton. Layer upon layer of these deposits piled up. The great weight of the deposits, plus bacteria, heat, and other natural forces, changed the chemical compounds in the plankton into natural gas and petroleum. The gas and oil flowed into openings in limestone, sandstone, and other kinds of rocks that are porous (full of tiny holes). Layers of solid rocks formed over the porous rocks and sealed the gas and oil beneath them. Later, movements in Earth’s crust caused the ancient seas to draw back, and dry land covered many gas and oil deposits.
Natural gas is referred to as a fossil fuel because it formed millions of years ago from the remains of living things. Petroleum and coal are also fossil fuels.
From well to user
Natural gas is found underground in various types of reservoir rock. This type of rock includes limestone, sandstone, carbonate, and other porous rocks. The pores are interconnected, and so gas can move through the rock. A dome of nonporous rock forms a cap over the reservoir rock, trapping the gas. The gas cannot escape unless well drillers open a hole through the solid rock or unless Earth’s surface shifts and cracks the cap. Natural gas is often found on top of oil deposits or dissolved in them, because the same natural processes formed both fuels.
Natural gas can also be also found in gas hydrates, solids that resemble wet snow. Gas hydrates, also called methane hydrates, are formed when water freezes in the presence of methane in the ocean depths. Under such conditions of extreme cold and pressure, the ice crystals form “cages” with gas molecules trapped inside. Gas hydrates also form readily in the extreme cold that occurs in the Arctic. The volume of gas stored in gas hydrates may be as great as 5,000 times the amount of conventional gas reserves known to exist in the world today. But retrieving the deposits is difficult because gas hydrates are not stable at normal surface temperatures and pressures. Without special equipment, the gas escapes from the ice when it reaches the surface. No one has yet found a cost-effective way to extract large quantities of fuel from gas hydrates.
Exploring for gas.
Modern exploration methods are constantly helping to uncover new reserves of natural gas. These methods show where there are geological formations that could hold gas. But they cannot indicate the actual presence of gas. The only sure way to find out if an area contains deposits of natural gas is to drill a well.
In proved areas, where gas or petroleum has already been found, about 75 percent of new wells bring in one of the two fuels. Drilling in unproved areas is called wildcatting. A wildcat well is drilled wherever a prospector believes that gas or oil may be present, in areas far from producing wells. About 70 percent of the wildcat wells produce some gas or oil. But many fail to produce enough fuel to pay for the cost of drilling them. Prospectors continue to drill in unproved areas because they keep part of the rights to deposits they discover. One successful wildcat well can more than make up for the cost of the unsuccessful ones.
In exploring for gas in unproved areas, prospectors rely on studies made by earth scientists called geologists and geophysicists. These studies involve maps, drilling records, and seismic measurements—that is, measurements of vibrations in the ground.
After selecting a promising site for a well, a geologist studies a detailed map of the features above and below Earth’s surface. The map enables the geologist to locate underground formations called traps, where natural gas and petroleum can accumulate. In addition, geologists try to determine whether there is reservoir rock underground. For more information on traps and reservoir rock, see Petroleum (How petroleum deposits form) .
Geologists may also study well logs. A well log is a record of the rock formations encountered during the drilling of a well. Well logs measure such characteristics as the porosity (presence of pores) and fluid content of the rock. By comparing well logs, geologists can determine how rocks differ from one area to another.
Geophysicists commonly use an exploration technique known as reflection seismology. In this technique, a loud noise, such as an explosion, is produced at or just below the surface. The sound waves that result travel into the ground and are reflected back to the surface by underground rock layers. In populated areas, a vibroseis truck, also called a thumper truck, may be used to produce the sound waves. A thumper truck has a huge vibrating pad that repeatedly strikes the ground. In offshore areas, sound waves are produced by sending a compressed-air discharge or an electronic pulse from a ship into the water.
Groups of geophones, which are similar to microphones, pick up the reflected sound waves. The pattern of the sound waves is recorded on an instrument called a seismograph (see Seismograph ). Sound waves change in amplitude (height) when they are reflected from rocks that contain gas. These changes in amplitude appear as irregularities, called bright spots, on the seismograph record.
Producing gas.
Drilling for gas involves the same methods as those used in drilling for oil. The most common method is rotary drilling. It is much like making a hole in wood with a carpenter’s drill. Another method, cable-tool drilling, is used chiefly to make shallow holes in soft rock. It is similar to punching a hole in wood with a hammer and a nail. For detailed information on drilling and other operations in the production of both gas and oil, see Petroleum (Drilling for petroleum) (Producing petroleum) .

Offshore wells are drilled in water that may be more than 10,000 feet (3,050 meters) deep. The North Sea, the Gulf Coast waters of the United States, and the waters off the western coast of Africa are among the richest offshore producing areas. Offshore drilling is usually much more productive than drilling on land, mainly because much less gas and oil have been taken from beneath the sea. But offshore drilling costs several times more. Instead of simply drilling down from land, offshore drillers must work from a barge, a movable rig, or a fixed platform.
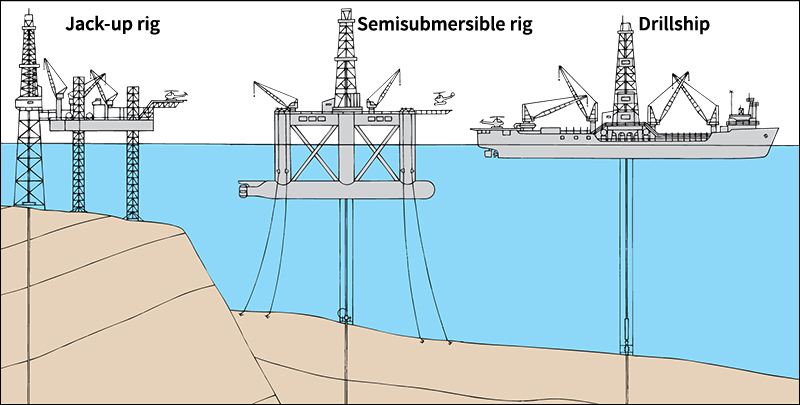
Transmitting and distributing gas.
The raw natural gas that flows from a well must be cleaned and treated before it is distributed. A pipe called a gathering line carries the gas from the well to an extraction unit, which removes such impurities as sand, sulfur, and water. The gas may then flow to nearby processing plants. The plants remove components that are not needed in the fuel and that can be easily condensed (converted to liquid), such as propane and gasoline. The processed natural gas is then fed into long-distance transmission pipelines, which carry it to communities along their routes. These pipelines are typically buried to minimize the risk of vandalism or accidental damage.
Gas is sent through transmission pipelines under high pressures—usually about 1,000 pounds per square inch (70 kilograms per square centimeter). The pressure drops along the route because of the friction of the gas against the pipe walls. The pressure also falls when communities remove gas. Compressor stations along the line restore high pressure and push the gas on to its farthest destination. Many lines have automatically operated stations that increase or decrease the pressure to meet the demands of various communities.
Gas usually travels through pipelines at about 15 miles (25 kilometers) per hour. Gas being pumped through pipelines from a well to a local distribution system thousands of miles or kilometers away can take several days to reach its destination.

Inspectors on foot and in airplanes check continually for conditions that might damage pipelines. After floods and heavy rains, for example, inspectors ensure that the pipelines remain covered with earth. In addition, instruments installed along the pipelines automatically report leaks and other faulty conditions.
In cities and towns, distribution lines carry the gas to consumers. There are two kinds of distribution lines—mains and individual service lines. Mains are large pipes connected to the transmission pipelines. Service lines are smaller pipes that branch out from the mains. The service lines carry the fuel sold by gas utility companies to homes, factories, restaurants, and other buildings.
Pure natural gas is odorless and would not be noticed if it leaked out. For this reason, gas utility companies add mercaptans, chemicals that contain sulfur, to the gas to give it a smell. For more information on pipelines, see the article on Pipeline.
Storing gas.
Consumers use much more gas in winter than in summer. Pipelines cannot carry enough gas to meet the demand for fuel on the coldest days. As a result, gas must be stored when the demand is low for use when the demand is high.
During the summer, many gas companies pump great quantities of natural gas back into the ground. Most underground storage areas are old gas or oil fields that are no longer productive, or other porous rock formations. In Colorado, an abandoned coal mine has been turned into a gas storage cavern. Ideal storage areas lie near pipelines, compressor stations, and—most important of all—large market areas.
If a gas company selects a nonproductive gas or oil field for storage, it must prepare the site to receive and hold gas. The company may have to repair and clean, or even replace, the well casings (large pipes put down the well to prevent it from caving in). The firm also may have to redrill the old well or drill a new one.
To find and prepare new underground storage sites, geologists and engineers use methods like those used in exploring and drilling for gas or oil. After a new storage field has been prepared and tested, huge machines pump in the gas under high pressure. When the company removes the stored gas to meet heavy demand in cold weather, it cleans and treats the fuel before sending it to the consumers.
Underground storage reservoirs are also important in the conservation of natural gas. Before the wide use of reservoirs, oil drillers often flared (burned) the natural gas found in an oil well to get rid of it during periods of low demand. Some of the great oil-producing countries in the Middle East still waste a large amount of gas in this way.
Natural gas is also stored through liquefaction—by changing it into a liquid. Methane becomes liquid when its temperature is lowered to about –260 °F (–162 °C). Raising the temperature returns the fuel to its gaseous form. Liquid natural gas (LNG) requires only about 1/600 of the storage space needed to store an equal quantity of natural gas. LNG can also be shipped overseas. For use in large volumes, LNG is more practical than LPG or other liquid gases because it has the same chemical makeup as natural gas. As a result, suppliers can easily switch between LNG and natural gas.
How gas is manufactured.
Gas is manufactured for its chemical by-products and for use as fuel. There are several types of manufactured gas. The most important are coke oven gas, also called coal gas, and acetylene.
Coke oven gas
is made by roasting coal. As the coal turns into coke, vapors consisting of many chemicals escape from the coal. The vapors are sent through water, which absorbs some of the unwanted chemicals. The rest of the gas bubbles up through the water. This gas may be further purified by various processes that remove chemical by-products. Coke oven gas has a much lower heating value than natural gas. Heating value refers to the amount of heat produced by a given amount of fuel when it is burned. See Coke oven gas.
Acetylene
is produced chiefly by creating a reaction between water and calcium carbide, a compound of calcium and carbon. Gas producers can also make acetylene by heating methane molecules to break them apart. Acetylene has a higher heating value than natural gas. It produces an extremely hot flame. Acetylene is used in welding and cutting metals. See Acetylene.
Other manufactured gases
include oil gas, producer gas, and water gas. Oil gas is made by spraying oil onto hot bricks to break apart the petroleum molecules. Producer gas is made by sending air slowly through a bed of hot coal or coke. The oxygen in the air combines with carbon in the coal, forming carbon monoxide. Water gas is made by forcing steam through a hot bed of coal or coke, forming carbon monoxide and hydrogen.
Gas and the environment
Harmful gases may be generated as by-products of the burning of natural gas. But burning other fossil fuels, and some alternative fuels, produces greater quantities of these gases, along with harmful particles of matter. Thus, natural gas burns relatively cleaner than these other fuels. Partly because of this environmental advantage, the demand for natural gas continues to increase.
Leaks in natural gas pipelines can release methane into the atmosphere, and burning natural gas produces carbon dioxide. Methane and carbon dioxide are often called greenhouse gases, because they trap heat from the sun and hold it near Earth’s surface. A build-up of greenhouse gases may result in global warming, an increase in the average temperature of Earth’s surface.
History of the gas industry
Early uses of natural gas.
The ancient Chinese were the first people known to use natural gas for industrial purposes. Thousands of years ago, they discovered natural gas deposits and learned to pipe the fuel through bamboo poles. They burned the gas to boil away salty water and collect the salt that remained.
As early as the A.D. 500’s, temples with “eternal” fires were built in western Asia, near what is now Baku, Azerbaijan. Worshipers came from as far as Persia and India to see the mysterious, continuous fires and wonder at the power of the temple priests. Secret pipes carried natural gas into the shrines from nearby rock fractures.
First uses of manufactured gas.
In 1609, Jan Baptista van Helmont, a Belgian chemist and physician, discovered manufactured gas. He found that a “spirit,” which he named gas, escaped from heated coal. In the late 1600’s, John Clayton, an English clergyman, roasted coal and collected the gas in animal bladders. He then punctured the bladders and lit the escaping gas.
In 1792, William Murdock, a British engineer, lighted his home with gas he made from coal. He installed gas lighting in his employers’ factory and foundry in 1803. By 1804, Murdock had installed 900 gaslights in cotton mills. He became known as the father of the gas industry. The work of Murdock and other experimenters interested Frederick Albert Winsor, a German businessman. Winsor decided to manufacture gas on a large scale. He learned the process from Murdock and obtained a British patent for manufacturing gas in 1804. In 1807, Winsor and his partners staged the first public street lighting with gas—along London’s Pall Mall. They formed the first gas company in 1812. The first gas company in the United States was established in 1817 in Baltimore to light that city’s streets.
Development of the natural gas industry
began in the United States. The earliest known discoveries of natural gas in the country occurred in 1775. That year, French missionaries in the Ohio Valley reported seeing “pillars of fire,” which probably were caused by seeping gas accidentally set on fire. Also in 1775, the American colonial leader George Washington saw a “burning spring”—flames rising from water—near what is now Charleston, West Virginia.
In 1821, mysterious bubbles appeared in a well being drilled for water at Fredonia, New York. The driller gave up his efforts. Soon afterward, on the same site, a gunsmith named William Aaron Hart completed the first natural gas well in the United States. It was 27 feet (8 meters) deep. Hart piped the gas to nearby buildings, and it was burned for lighting. Another shallow natural gas well was drilled near Westfield, New York, in 1826.
The first company known to have distributed natural gas was formed in Fredonia in 1865. By then, about 300 U.S. companies were distributing manufactured gas. Oil was discovered near Titusville, Pennsylvania, in 1859, and natural gas development was nearly forgotten in the oil rush that followed. The gas found in the oil fields lacked both markets and pipeline systems.

The first “long-distance” pipeline was completed in 1872. This 25-mile (40-kilometer) wooden pipeline carried natural gas to hundreds of consumers in Rochester, New York. Also in 1872, the first iron pipeline for natural gas began carrying the fuel 51/2 miles (9 kilometers) to Titusville. This pipeline delivered 4 million cubic feet (110,000 cubic meters) of gas daily to about 250 consumers.
In 1879, the American inventor Thomas A. Edison developed the first practical incandescent lamp. This development and the introduction of electric lighting nearly destroyed the gas industry. However, the industry started to grow again as more and more consumers turned to manufactured gas for cooking and water heating. Meanwhile, natural gas development remained at a standstill. However, during the early 1900’s, huge gas reserves were discovered in Texas, Louisiana, and Oklahoma. From 1906 to 1920, natural gas production in the United States more than doubled, to 800 billion cubic feet (23 billion cubic meters) a year. The lack of long-distance pipelines held back further growth in the industry. By 1925, there were about 31/2 million natural gas consumers, but all were within a few hundred miles or kilometers of the gas fields.
The natural gas industry began to expand rapidly in the late 1920’s with the introduction of seamless, electrically welded steel pipe. This pipe was stronger than earlier pipe. It could carry gas under higher pressures and, therefore, in greater quantities. With the new pipe, companies could profitably build lines over 1,000 miles (1,600 kilometers) long. The earliest of such lines delivered gas from Texas fields to cities in the midwestern United States.
Until the 1960’s, large quantities of natural gas were not available in many industrialized countries, and manufactured gas was used widely. In the 1960’s, the development of newly discovered gas fields led to the rapid expansion of Europe’s natural gas industry. This expansion was especially fast in the Soviet Union and the Netherlands. The world’s largest known gas field was discovered in the Soviet Union in 1966. The United Kingdom began to produce much natural gas from deposits found under the North Sea in the mid-1960’s.
The modern gas industry.
Modern methods of exploring for natural gas have led to the greatest world supply of gas in history. A number of nations that had depended largely or entirely on manufactured gas have discovered large deposits of natural gas and are switching to the cheaper fuel. Producing countries are exporting an increasing amount of natural gas by new pipelines or in liquid form by tanker ships.
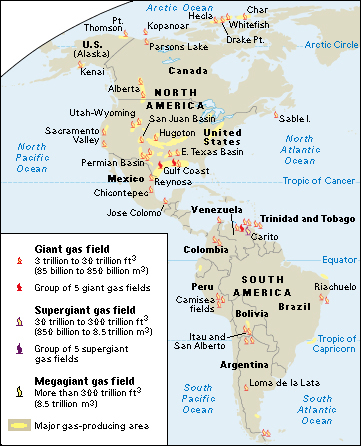
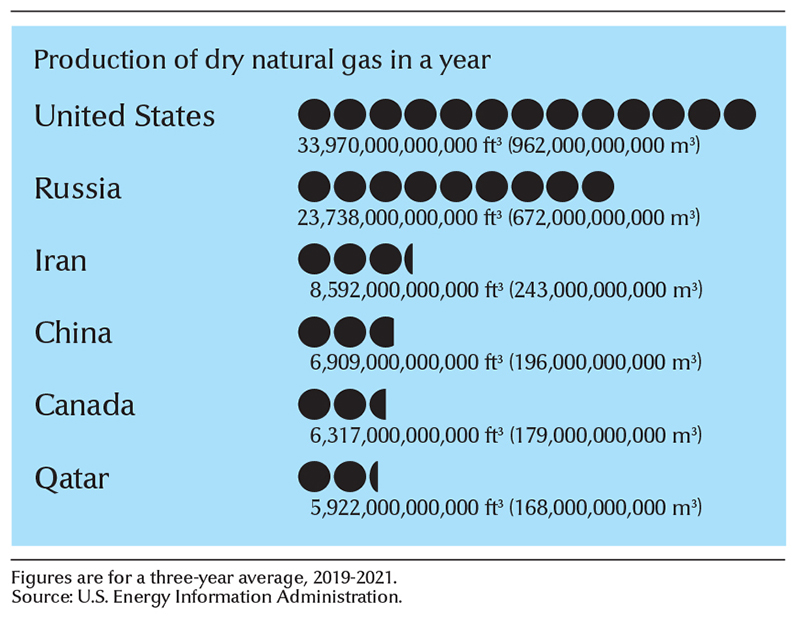
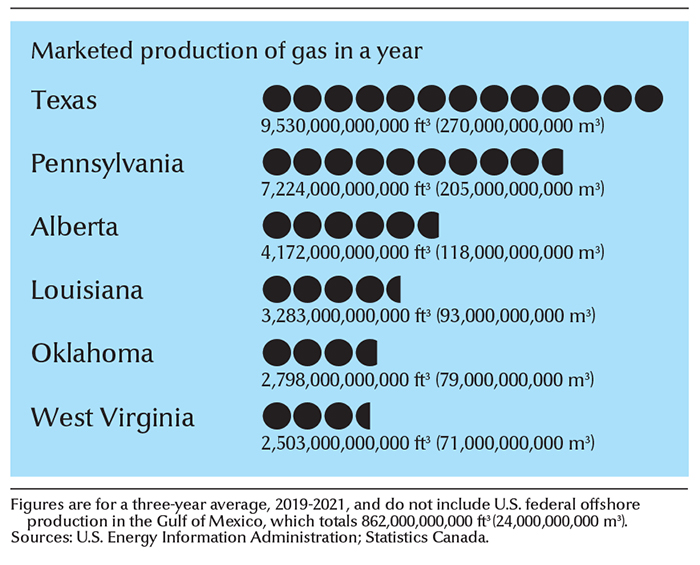
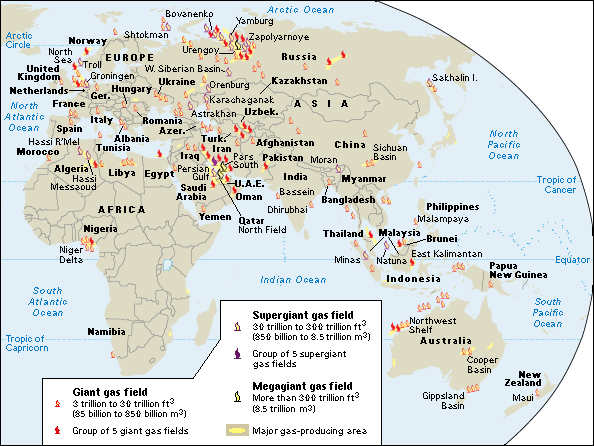
The largest known natural gas reserves are in the Middle East and Russia. The United States and Russia lead the nations, by far, in natural gas production. Other leading natural gas-producing countries include Australia, Canada, China, Iran, and Qatar. Texas leads the United States in natural gas production. A large amount of U.S. gas is produced offshore in the Gulf of Mexico. Alberta produces more natural gas than any other Canadian province.
The gas industry is developing more efficient ways to use natural gas. See the Gas in industry section of this article for information on these advances. A device under development is a gas fuel cell. It produces electric power chemically, using methane from natural gas.
The problem of air pollution has created interest in natural gas as a transportation fuel. Natural gas produces lower amounts of dangerous emissions when burned than gasoline and diesel fuel do. Gas is being used, mostly on an experimental basis, to power some automobiles, trucks, and ships.
In some regions, natural gas consumption exceeds the amount made available from the discovery of new reserves. Some people fear that the demand for gas might soon exceed the world’s supply. The gas industry has been exploring for additional sources of natural gas. It has also sought new and better ways of producing clean-burning gas from coal (see Coal (Coal research) ).
