Germanium, << juhr MAY nee uhm, >> a chemical element, is a hard, brittle, grayish-white metalloid. It is obtained during the refining of copper, zinc, and lead ores.
Germanium is one of the most widely used materials known as semiconductors (see Semiconductor ). Germanium is a good semiconductor because it does not have strong metallic properties. Industry uses germanium in such semiconducting devices as diodes and solar batteries. It is also a component of various infrared optics devices that are used in telephone lines, data transmission lines, and computer cables.
The addition of trace amounts of germanium to a lead-acid storage battery can help the battery last longer. Germanium oxide, a germanium compound, is used in medicine.
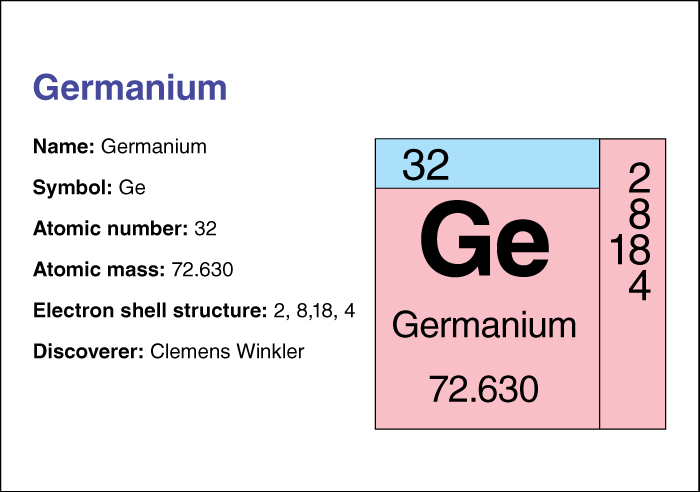
Germanium has the chemical symbol Ge. Its atomic number (number of protons in its nucleus) is 32. Its relative atomic mass is 72.64. An element’s relative atomic mass equals its mass (amount of matter) divided by 1/12 of the mass of carbon 12, the most abundant form of carbon. Germanium melts at 937.4 °C and boils at 2830 °C. The German chemist Clemens Winkler discovered germanium in 1886.
For information on the position of germanium on the periodic table, see the article Periodic table .
