Iodine is a nonmetallic chemical element . It is a member of the halogen family of elements. At ordinary temperatures, iodine is a shiny bluish-black solid with an irritating odor. When heated, it usually sublimes —that is, it changes from a solid directly into a vapor . Iodine vapor is purple. The word iodine comes from a Greek word meaning purple.
Uses of iodine.
Plants and animals need traces of iodine for normal growth. In the human body, the thyroid gland , which is located in the neck, uses iodine to produce the hormone thyroxine. Thyroxine controls the body’s rate of physical and mental development. Iodine deficiency can hinder growth and can also produce goiter , an enlargement of the thyroid gland. For these reasons, manufacturers add small amounts of potassium iodide or sodium iodide to table salt in areas where iodine levels in food and water are low. Pure iodine is poisonous if swallowed.
Iodine and its compounds have important commercial uses. For example, silver iodide serves as the principal light-sensitive substance in photographic film. Commercial bakers use another compound, sodium iodate, to improve the quality of bread made from certain kinds of flour . Iodine and iodine compounds are used in purifying water and as disinfectants . For many years, people have used a solution of iodine in alcohol, called tincture of iodine, as a first-aid antiseptic . But this solution can irritate wounds. As a result, instead of tincture of iodine, many people now use more complex iodine compounds called iodophors.
Sources of iodine.
The chief source of iodine is brine (very salty water) that contains sodium iodide and potassium iodide. Such brine is obtained from wells in petroleum fields and natural gas fields. Another source of iodine is the mineral lautarite, in which iodine forms the compound calcium iodate. Lautarite is found chiefly in nitrate deposits in Chile . Seawater contains iodine compounds, but only in very low concentrations. But high concentrations form in many marine organisms. Certain seaweeds called kelps contain especially high concentrations of iodine. They were one of the earliest sources of the element.
Properties of iodine.
Like the other halogens, iodine can oxidize (gain electrons from) other substances and combine with them to form chemical compounds. However, iodine oxidizes other substances less readily than do the lighter halogens bromine , chlorine , and fluorine . On the other hand, iodine is more likely than these halogens to lose electrons and so become oxidized itself. Oxidized iodine can combine with bromine, chlorine, fluorine, or oxygen .
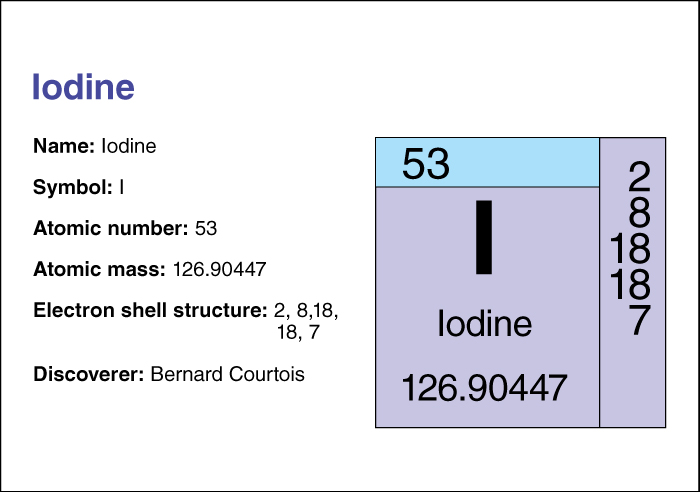
Iodine has the chemical symbol I. Its atomic number (number of protons in its nucleus) is 53. Its relative atomic mass is 126.90447. An element’s relative atomic mass equals its mass (amount of matter) divided by 1/12 of the mass of carbon 12, the most abundant form of carbon . Iodine melts at 113.60 °C and boils at 185.24 °C. For information on the position of iodine on the periodic table, see the article Periodic table .
Iodine was discovered by the French chemist Bernard Courtois , who found it in the ashes of burnt seaweed in 1811. In 1814, the French chemist Joseph L. Gay-Lussac became the first person to recognize iodine as a chemical element.
